L-Cysteine hydrochloride monohydrateCAS# 7048-04-6 |
Quality Control & MSDS
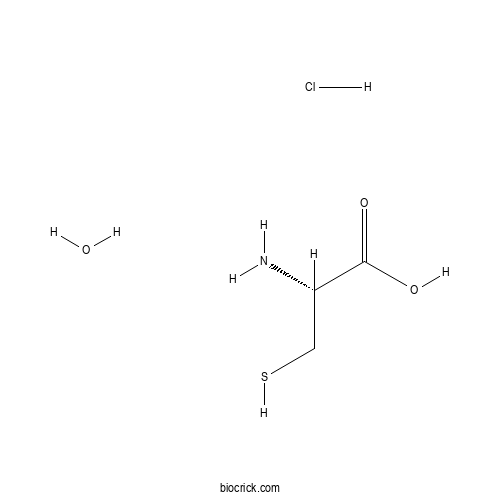
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7048-04-6 | SDF | Download SDF |
| PubChem ID | 23462.0 | Appearance | Powder |
| Formula | C3H10ClNO3S | M.Wt | 175.63 |
| Type of Compound | Amino Acids | Storage | Desiccate at -20°C |
| Synonyms | L-cysteine hcl monohydrate; H-Cys-OH.HCl.H2O; H-Cys-OH.HCl.H_2O | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R)-2-amino-3-sulfanylpropanoic acid;hydrate;hydrochloride | ||
| SMILES | C(C(C(=O)O)N)S.O.Cl | ||
| Standard InChIKey | QIJRTFXNRTXDIP-JIZZDEOASA-N | ||
| Standard InChI | InChI=1S/C3H7NO2S.ClH.H2O/c4-2(1-7)3(5)6;;/h2,7H,1,4H2,(H,5,6);1H;1H2/t2-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

L-Cysteine hydrochloride monohydrate Dilution Calculator

L-Cysteine hydrochloride monohydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6938 mL | 28.4689 mL | 56.9379 mL | 113.8758 mL | 142.3447 mL |
| 5 mM | 1.1388 mL | 5.6938 mL | 11.3876 mL | 22.7752 mL | 28.4689 mL |
| 10 mM | 0.5694 mL | 2.8469 mL | 5.6938 mL | 11.3876 mL | 14.2345 mL |
| 50 mM | 0.1139 mL | 0.5694 mL | 1.1388 mL | 2.2775 mL | 2.8469 mL |
| 100 mM | 0.0569 mL | 0.2847 mL | 0.5694 mL | 1.1388 mL | 1.4234 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Uvarimacrophin A
Catalog No.:BCX1601
CAS No.:167425-75-4
- Isobergaptol
Catalog No.:BCX1600
CAS No.:21339-45-7
- Schindilactone A
Catalog No.:BCX1599
CAS No.:943239-45-0
- Apigenin-7-O-glucuronide-6'-ethyl ester
Catalog No.:BCX1598
CAS No.:62268-42-2
- 14-Formyldihydrorutaecarpine
Catalog No.:BCX1597
CAS No.:68353-23-1
- Arisanlactone D
Catalog No.:BCX1596
CAS No.:1234186-01-6
- Oleaside B
Catalog No.:BCX1595
CAS No.:71699-08-6
- Vinaginsenoside R7
Catalog No.:BCX1594
CAS No.:156009-83-5
- Telephiose C
Catalog No.:BCX1593
CAS No.:297749-31-6
- Daphneticin
Catalog No.:BCX1592
CAS No.:83327-22-4
- Wuweizidilactone A
Catalog No.:BCX1591
CAS No.:945610-99-1
- Telephiose A
Catalog No.:BCX1590
CAS No.:297749-29-2
- Orientin-2''-O-p-trans-coumarate
Catalog No.:BCX1603
CAS No.:1229437-75-5
- L-Glutamic acid hydrochloride
Catalog No.:BCX1604
CAS No.:138-15-8
- L-Homoarginine hydrochloride
Catalog No.:BCX1605
CAS No.:1483-01-8
- 4-(3-oxobutyl)phenyl 6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-beta-D-glucopyranoside
Catalog No.:BCX1606
CAS No.:1609583-04-1
- 3-(4-methoxyphenyl)-3,4-dihydro-2H-chromen-7-ol
Catalog No.:BCX1607
CAS No.:10499-17-9
- Nudol
Catalog No.:BCX1608
CAS No.:86630-46-8
- 4-Methoxyphenyl-beta-galactoside
Catalog No.:BCX1609
CAS No.:3150-20-7
- Spinorhamnoside
Catalog No.:BCX1610
CAS No.:864271-19-2
- Ophiopogonin A
Catalog No.:BCX1611
CAS No.:11054-24-3
- Rathbunioside R1
Catalog No.:BCX1612
CAS No.:350689-78-0
- Mesatlantin C
Catalog No.:BCX1613
CAS No.:137624-14-7
- Ethyl-beta-D- glucoside
Catalog No.:BCX1614
CAS No.:19467-01-7
Green Synthesis of Crystalline Silica from Sugarcane Bagasse Ash: Physico-Chemical Properties.[Pubmed:35808020]
Nanomaterials (Basel). 2022 Jun 25;12(13):2184.
Sugarcane bagasse South Africa is an agricultural waste that poses many environmental and human health problems. Sugarcane bagasse dumps attract many insects that harm the health of the population and cause many diseases. Sugarcane ash is a naturally renewable source of silica. This study presents for the first time the extraction of nanosilica from sugar cane bagasse ash using L-Cysteine hydrochloride monohydrate acid and Tetrapropylammonium Hydroxide. The structural, morphological, and chemical properties of the extracted silica nanoparticles was cross examined using XRD, FTIR, SEM, and TGA. SEM analysis presents agglomerates of irregular sizes. It is possible to observe the structure of nanosilica formed by the presence of agglomerates of irregular shapes, as well as the presence of some spherical particles distributed in the structure. XRD analysis has revealed 2theta angles at 20, 26, 36, 39, 50, and 59 which shows that each peak on the xrd pattern is indicative of certain crystalline cubic phases of nanosilica, similar to results reported in the literature by Jagadesh et al. in 2015. The crystallite size estimated by the Scherrer equation based on the aforementioned peaks for ca-silica and L-cys-silica for the extracted particles had an average diameter of 26 nm and 29 nm, respectively. Furthermore, it showed a specific surface area of 21.6511 m(2)/g and 116.005 m(2)/g for ca-silica and L-cys silica, respectively. The Infrared (IR) spectra showed peaks at 461.231 cm(-1), 787.381 cm(-1) and 1045.99 cm(-1) which corresponds to the Si~O~Si bending vibration, the Si~O~Si stretch vibration, and the Si~O~Si stretching vibration, respectively. This confirms the successful extraction of nanosilica from sugar cane bagasse ash. TGA analysis has revealed that the as received sugarcane bagasse has high loss on ignition (LOI) of 18%, corresponding to the presence of the unburnt or partial burnt particles, similar to results reported by Yadav et al. This study has shown that sugar cane bagasse ash is a natural resource of silica which should be harnessed for industrial purposes in south Africa.
Safety and efficacy of l-cysteine monohydrochloride monohydrate produced by fermentation using Escherichia coli KCCM 80109 and Escherichia coli KCCM 80197 for all animal species.[Pubmed:32874303]
EFSA J. 2020 Apr 30;18(4):e06101.
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of l-cysteine monohydrochloride monohydrate produced by fermentation using two non-genetically modified strains of Escherichia coli K12 (E. coli KCCM 80109 and E. coli KCCM 80197) as a flavouring additive for all animal species. No safety concerns are derived from the use of these strains as production strains of the additive. The FEEDAP Panel concludes that the use of L-Cysteine hydrochloride monohydrate produced by E. coli KCCM 80109 and KCCM 80197 at concentrations up to 25 mg/kg complete feed is safe for the target species, for the consumer and for the environment. The product is proposed to be classified as respiratory irritant; however, exposure by inhalation is unlikely. Based on the results of the studies provided, it should be classified as skin irritant and that it causes serious eye damage. L-Cysteine hydrochloride monohydrate is not a dermal sensitiser. Since L-Cysteine hydrochloride monohydrate is used in food as flavourings, it is to be expected that it can provide a similar function in feed and no further demonstration of efficacy is necessary when used at concentrations up to 25 mg/kg complete feed and the corresponding concentration in water.
Safety and efficacy of l-cysteine hydrochloride monohydrate produced by fermentation using Escherichia coli KCCM 80180 and Escherichia coli KCCM 80181 as a flavouring additive for all animal species.[Pubmed:32874218]
EFSA J. 2020 Feb 10;18(2):e06003.
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of l-cysteine monohydrochloride monohydrate produced by fermentation using two genetically modified strains of Escherichia coli K12 (Escherichia coli KCCM 80180 and Escherichia coli KCCM 80181) as a flavouring additive for all animal species. No safety concerns are derived from the use of these strains as production strains of the additive. The FEEDAP Panel concludes that L-Cysteine hydrochloride monohydrate, produced by E.coli KCCM 80180 and KCCM 80181 at concentrations up to 25 mg/kg complete feed, is safe for the target species, for the consumer and for the environment. The product is proposed to be classified as respiratory irritant; however, exposure by inhalation is unlikely. L-Cysteine hydrochloride monohydrate produced by E.coli KCCM 80180 and E.coli KCCM 80181 was shown to be a skin and eye irritant but not a skin sensitiser. Since L-Cysteine hydrochloride monohydrate is used in food as flavouring, it is to be expected that it can provide a similar function in feed and no further demonstration of efficacy is necessary when used at concentrations up to 25 mg/kg complete feed and the corresponding concentration in water.


