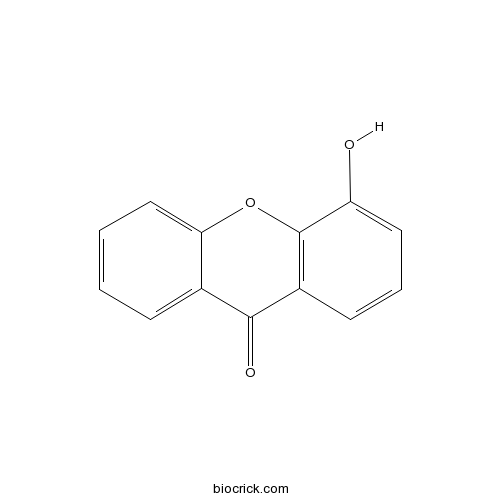
4-HydroxyxanthoneCAS# 14686-63-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14686-63-6 | SDF | Download SDF |
| PubChem ID | 611428 | Appearance | Powder |
| Formula | C13H8O3 | M.Wt | 212.2 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-hydroxyxanthen-9-one | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C3=C(O2)C(=CC=C3)O | ||
| Standard InChIKey | KBQFPPUAIJHDCO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H8O3/c14-10-6-3-5-9-12(15)8-4-1-2-7-11(8)16-13(9)10/h1-7,14H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Hydroxyxanthone Dilution Calculator

4-Hydroxyxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7125 mL | 23.5627 mL | 47.1254 mL | 94.2507 mL | 117.8134 mL |
| 5 mM | 0.9425 mL | 4.7125 mL | 9.4251 mL | 18.8501 mL | 23.5627 mL |
| 10 mM | 0.4713 mL | 2.3563 mL | 4.7125 mL | 9.4251 mL | 11.7813 mL |
| 50 mM | 0.0943 mL | 0.4713 mL | 0.9425 mL | 1.885 mL | 2.3563 mL |
| 100 mM | 0.0471 mL | 0.2356 mL | 0.4713 mL | 0.9425 mL | 1.1781 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Macluraxanthone
Catalog No.:BCN9421
CAS No.:5848-14-6
- Deoxymikanolide
Catalog No.:BCN9420
CAS No.:23753-57-3
- 4,5-Dihydropiperlonguminine
Catalog No.:BCN9419
CAS No.:23512-53-0
- Dihydromikanolide
Catalog No.:BCN9418
CAS No.:23758-04-5
- 2,6-Dihydroxyxanthone
Catalog No.:BCN9417
CAS No.:838-11-9
- Minisecolide C
Catalog No.:BCN9416
CAS No.:1967030-77-8
- Withaphysalin A
Catalog No.:BCN9415
CAS No.:57423-72-0
- Withaphysalin E
Catalog No.:BCN9414
CAS No.:118985-24-3
- Withaphysalin S
Catalog No.:BCN9413
CAS No.:949172-13-8
- Methyl (9Z,11E)-13-hydroxyoctadeca-9,11-dienoate
Catalog No.:BCN9412
CAS No.:109837-85-6
- Physaminimin C
Catalog No.:BCN9411
CAS No.:1582259-03-7
- Physaminimin D
Catalog No.:BCN9410
CAS No.:1582259-05-9
- 3-(Hydroxyacetyl)indole
Catalog No.:BCN9423
CAS No.:2400-51-3
- Minisecolide D
Catalog No.:BCN9424
CAS No.:1967030-78-9
- Lipodeoxyaconitine
Catalog No.:BCN9425
CAS No.:244190-83-8
- Lipoaconitine
Catalog No.:BCN9426
CAS No.:81941-14-2
- 3-epi-Dihydroscandenolide
Catalog No.:BCN9427
CAS No.:1137951-08-6
- cis-Shegansu B
Catalog No.:BCN9428
CAS No.:865474-99-3
- Methyl 2,4,6-trihydroxybenzoate
Catalog No.:BCN9429
CAS No.:3147-39-5
- Elatoside E
Catalog No.:BCN9430
CAS No.:156980-30-2
- 1H-Indole-3-carbaldehyde
Catalog No.:BCN9431
CAS No.:487-89-8
- Scandenolide
Catalog No.:BCN9432
CAS No.:23758-16-9
- Neoorthosiphol A
Catalog No.:BCN9433
CAS No.:243448-72-8
- Orthosiphol B
Catalog No.:BCN9434
CAS No.:144078-08-0
Schomburgkixanthone, a novel bixanthone from the twigs of Garcinia schomburgkiana.[Pubmed:31984782]
Nat Prod Res. 2020 Jan 25:1-6.
A novel bixanthone, named schomburgkixanthone (1), was isolated from the twigs of Garcinia schomburgkiana, along with six known compounds, griffipavixanthone (2), 4-Hydroxyxanthone (3), 2-hydroxyxanthone (4), 1,6-dihydroxyxanthone (5), 1,7-dihydroxyxanthone (6), and 1,3,5-trihydroxyxanthone (7). The structure of 1 was identified by the application of NMR and MS data analyses and comparison with previous reports. Compound 1 showed the most powerful inhibition of rat intestinal alpha-glucosidase, with IC50 values of 0.79 for maltase and 1.81 mM for sucrase. Compound 2 most strongly inhibited sucrase, with an IC50 value of 4.58 mM.
Antiproliferative xanthone derivatives from Calophyllum inophyllum and Calophyllum soulattri.[Pubmed:25730799]
Pak J Pharm Sci. 2015 Mar;28(2):425-9.
Structure-activity relationships of eleven xanthones were comparatively predicted for four cancer cell lines after the compounds were subjected to antiproliferative assay against B-lymphocyte cells (Raji), colon carcinoma cells (LS174T), human neuroblastoma cells (IMR-32) and skin carcinoma cells (SK-MEL-28). The eleven chemical constituents were obtained naturally from the stem bark of Calophyllum inophyllum and Calophyllum soulattri. Inophinnin (1) and inophinone (2) were isolated from Calophyllum inophyllum while soulattrin (3) and phylattrin (4) were found from Calophyllum soulattri. The other xanthones were from both Calophyllum sp. and they are pyranojacareubin (5), rheediaxanthone A (6), macluraxanthone (7), 4-Hydroxyxanthone (8), caloxanthone C (9), brasixanthone B (10) and trapezifolixanthone (11). Compound 3 was found to be the most cytotoxic towards all the cancer cell lines with an IC50 value of 1.25mug/mL while the simplest xanthone, compound 8 was inactive.
Calophyllum inophyllum and Calophyllum soulattri source of anti-proliferative xanthones and their structure-activity relationships.[Pubmed:25229947]
Nat Prod Res. 2015;29(1):98-101.
Extensive chromatographic isolation and purification of the extracts of the stem bark of Calophyllum inophyllum and Calophyllum soulattri have resulted in 11 xanthones. C. inophyllum gave inophinnin (1), inophinone (2), pyranojacareubin (5), rheediaxanthone A (6), macluraxanthone (7) and 4-Hydroxyxanthone (8), while C. soulattri afforded soulattrin (3), phylattrin (4), caloxanthone C (9), brasixanthone B (10) and trapezifolixanthone (11). The structures of these compounds were determined on the basis of spectroscopic analyses such as 1D and 2D NMR, GC-MS, IR and UV. Cytotoxicity screening (MTT assay) carried out in vitro on all the xanthones using five human cancer cell lines indicated good activities for some of these xanthones. The structure-activity relationship study revealed that the inhibitory activities exhibited by these xanthone derivatives to be closely related to the existence and nature of the pyrano and the prenyl substituent groups on their skeleton.
Mechanism of the vasodilator effect of mono-oxygenated xanthones: a structure-activity relationship study.[Pubmed:24037589]
Planta Med. 2013 Nov;79(16):1495-500.
The present study characterized the mechanisms involved in the vasodilator effect of two mono-oxygenated xanthones, 4-Hydroxyxanthone and 4-methoxyxanthone. 9-Xanthenone, the base structure of xanthones, was used for comparison. 4-Hydroxyxanthone and 9-xanthenone induced a concentration-dependent and endothelium-independent vasodilator effect in arteries precontracted with phenylephrine (0.1 micromol . L-1) or KCl (50 mmol . L-1). 4-Methoxyxanthone induced a concentration-dependent vasodilator effect in arteries precontracted with phenylephrine, which was partially endothelium-dependent, and involved production of nitric oxide. In endothelium-denuded arteries precontracted with KCl, the vasodilator effect of 4-methoxyxanthone was abolished. The vasodilator effect of 4-Hydroxyxanthone (96.22 +/- 2.10 %) and 4-methoxyxanthone (96.57 +/- 12.40 %) was significantly higher than observed with 9-xanthenone (53.63 +/- 8.31 %). The presence of an oxygenated radical in position 4 made 4-Hydroxyxanthone (pIC50 = 4.45 +/- 0.07) and 4-methoxyxanthone (pIC50 = 5.04 +/- 0.09) more potent as a vasodilator than 9-xanthenone (pIC50 = 3.92 +/- 0.16). In addition, 4-methoxyxanthone was more potent than the other two xanthones. Ca2+ transients in vascular smooth muscle cells elicited by high K+ were abolished by 4-Hydroxyxanthone and 9-xanthenone. The endothelium-independent effect of 4-methoxyxanthone was abolished by inhibition of K+ channels by tetraethylammonium. The current work shows that an oxygenated group in position 4 is essential to achieve Emax and to increase the potency of xanthones as vasodilators. Substitution of an OH by OCH3 in position 4 increases the potency of the vasodilator effect and changes the underling mechanism of action from the blockade of L-type calcium channels to an increase in NO production and activation of K+ channels.
A new furanoxanthone from the stem bark of Calophyllum inophyllum.[Pubmed:21972812]
J Asian Nat Prod Res. 2011 Oct;13(10):956-60.
The stem bark extracts of Calophyllum inophyllum furnished one new furanoxanthone, inophinnin (1), in addition to inophyllin A (2), macluraxanthone (3), pyranojacareubin (4), 4-Hydroxyxanthone, friedelin, stigmasterol, and betulinic acid. The structures of these compounds were determined by spectroscopic analysis of 1D and 2D NMR spectral data ((1)H, (13)C, DEPT, COSY, HMQC, and HMBC) while EI-MS gave the molecular mass. The new xanthone, inophinnin (1), exhibited some anti-inflammatory activity in nitric oxide assay.
Vasodilator and antioxidant effect of xanthones isolated from Brazilian medicinal plants.[Pubmed:19090455]
Planta Med. 2009 Feb;75(2):145-8.
Vasorelaxant and antioxidant activities are important in the therapy for cardiovascular diseases. We aimed at investigating the vasorelaxant and antioxidant activities of six xanthones isolated from Brazilian medicinal plants. Xanthone ( 1), 1-hydroxyxanthone ( 2), 4-Hydroxyxanthone ( 3), 1-hydroxy-8-methoxyxanthone ( 4), 1,3-dihydroxy-7-methoxyxanthone ( 5) and 2,6,8-trihydroxy-1-methoxyxanthone ( 6) induced concentration-dependent vasorelaxant effects in endothelium-intact mice aortic rings. The presence of a hydroxy group in position 1 seemed to decrease the vasodilator effect while a hydroxy in position 4 and an increased number of hydroxy groups improved the vasorelaxatory potential of xanthones. All xanthones showed antioxidant activity but their potencies did not correlate with the vasodilator effect. Our results suggest that the tested xanthones are potentially vasorelaxant and antioxidant compounds but the two activities are not interrelated.
[Studies on flavonoids from stems and leaves of Calophyllum inophyllum].[Pubmed:17608221]
Zhongguo Zhong Yao Za Zhi. 2007 Apr;32(8):692-4.
OBJECTIVE: To study the chemical constituents from the stems and leaves of Calophyllum inophyllum. METHOD: The compounds were isolated by column chromatography on silica gel, Sephadex LH-20 and preparative TLC. Their structures were elucidated by chemical methods and NMR, MS spectroscopic data. RESULT: Nine compounds were identified as 2-hydroxyxanthone (1), 4-Hydroxyxanthone (2), 1, 5-dihydroxyxanthone (3), 1, 7-dihydroxyxanthone (4), 1, 3, 5-trihydroxy-2-methoxyxanthone (5), 6-deoxyjacareubin (6), amentoflavone (7), kaempferol-3-O-alpha-L-rhamnoside (8) and quercetin-3-O-alpha-L-rhamnoside (9). CONCLUSION: Compounds 8 and 9 were isolated from the genus Calophyllum and compounds 1, 2, 4-6 were isolated from this plant for the first time.
Xanthones from the stems of Securidaca inappendiculata.[Pubmed:11738416]
Phytochemistry. 2001 Dec;58(8):1245-9.
From the stems of Securidaca inappendiculata, securixanthones A (1,3,7-trihydroxy-2,8-dimethoxyxanthone) and B (3,7-dimethoxy-4-Hydroxyxanthone) along with ten known xanthones were isolated. Their structures were elucidated by analysis chemical and spectroscopic evidence, and the chemotaxonomic significance of these findings are also discussed.


