4-Methoxycinnamic acidCAS# 830-09-1 |
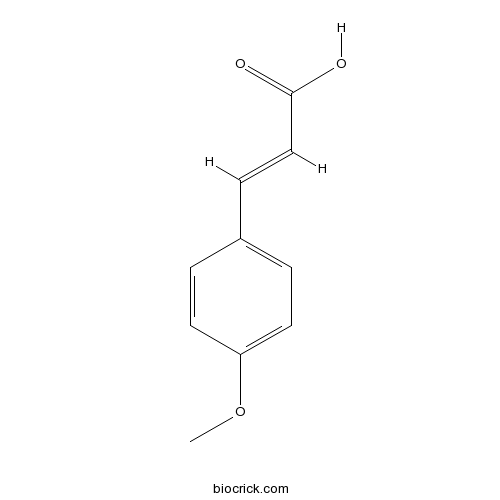
- trans-4-Methoxycinnamic acid
Catalog No.:BCX0620
CAS No.:943-89-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 830-09-1 | SDF | Download SDF |
| PubChem ID | 699414 | Appearance | White powder |
| Formula | C10H10O3 | M.Wt | 178.18 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(4-methoxyphenyl)prop-2-enoic acid | ||
| SMILES | COC1=CC=C(C=C1)C=CC(=O)O | ||
| Standard InChIKey | AFDXODALSZRGIH-QPJJXVBHSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4-Methoxycinnamic acid is a photosensitive compound, it shows various pharmacologic actions such as anti-cancer, hepatoprotective and antihyperglycemic activities, it also can stimulate insulin secretion from pancreatic β-cells by increasing Ca2+ influx via the L-type Ca2+ channels, but not through the closure of ATP-sensitive K+ channels. 4-Methoxycinnamic acid can strongly inhibit the diphenolase activity of mushroom tyrosinase, with the IC 50 value of 0.42 mM, and the inhibition is reversible. |
| Targets | Calcium Channel | ATPase | Potassium Channel | P450 (e.g. CYP17) | NADPH-oxidase |
| In vitro | Natural products modulate Shigella-host-cell interaction.[Pubmed: 21719574]J Med Microbiol. 2011 Nov;60(Pt 11):1626-32.This study focused on identifying possible new options derived from natural sources for the treatment of bacterial infections. Several natural products were investigated for their potential in modulating Shigella-host-cell interactions. Photosensitive semiconductor nanocrystals, photosensitive composition comprising semiconductor nanocrystals and method for forming semiconductor nanocrystal pattern using the same[Reference: WebLink]US 8758864 B2[P]. 2014.4. The organic-inorganic hybrid electroluminescent device according to claim 1, wherein the compound containing a photosensitive functional group is selected from a group consisting of methacrylic acid, crotonic acid, vinylacetic acid, tiglic acid, 3,3-dimethylacrylic acid, trans-2-pentenoic acid, 4-pentenoic acid, trans-2-methyl-2-pentenoic acid, 2,2-dimethyl-4-pentenoic acid, trans-2-hexenoic acid, trans-3-hexenoic acid, 2-ethyl-2-hexenoic acid, 6-heptenoic acid, 2-octenoic acid, citronellic acid, undecylenic acid, myristoleic acid, palmitoleic acid, oleic acid, elaidic acid, cis-11-elcosenoic acid, euric acid, nervonic acid, trans-2,4-pentadienoic acid, 2,4-hexadienoic acid, 2,6-heptadienoic acid, geranic acid, linoleic acid, 11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, arachidonic acid, cis-5,8,11,14,17-eicosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, fumaric acid, maleic acid, itaconic acid, ciraconic acid, mesaconic acid, trans-glutaconic acid, trans-beta-hydromuconic acid, trans-traumatic acid, trans-muconic acid, cis-aconitic acid, trans-aconitic acid, cis-3-chloroacrylic acid, trans-3-chloroacrylic acid, 2-bromoacrylic acid, 2-(trifluoromethyl)acryl-ic acid, trans-styrylacetic acid, trans-cinnamic acid, alpha.-methylcinnamic acid, 2-methylcinnamic acid, 2-fluorocinnamic acid, 2-(trifluoromethyl)cinnamic acid, 2-chlorocinnamic acid, 2-methoxycinnamic acid, 2-hydroxycinnamic acid, 2-nitrocinnamic acid, 2-carboxycinnamic acid, trans-3-fluorocinnamic acid, 3-(trifluoromethyl)cinnamic acid, 3-chlorocinnamic acid, 3-bromocinnamic acid, 3-methoxycinnamic acid, 3-hydroxycinnamic acid, 3-nitrocinnamic acid, 4-methylcinnamic acid, 4-fluorocinnamic acid, trans-4-(trifluoromethyl)-cinnamic acid, 4-chlorocinnamic acid, 4-bromocinnamic acid, 4-Methoxycinnamic acid, 4-hydroxycinnamic acid, 4-nitrocinnamic acid, 3,3-dimethoxycinnamic acid, 4-vinylbenzoic acid, allyl methyl sulfide, allyl disulfide, diallyl amine, oleylamine, 3-amino-1-propanol vinyl ether, 4-chlorocinnamonitrile, 4-methoxycinnamonitrile, 3,4-dimethoxycinnamonitrile, 4-dimethylaminocinnamonitrile, acrylonitrile, allyl cyanide, crotononitrile, methacrylonitrile, cis-2-pentenenitrile, trans-3-pentenenitrile, 3,7-dimethyl-2,6-octadienenitrile, and 1,4-dicyano-2-butene. |
| In vivo | Protective effect of p-methoxycinnamic acid, an active phenolic acid against 1,2-dimethylhydrazine-induced colon carcinogenesis: modulating biotransforming bacterial enzymes and xenobiotic metabolizing enzymes.[Pubmed: 24908112 ]Mol Cell Biochem. 2014 Sep;394(1-2):187-98.Objective of the study is to evaluate the modifying potential of p-methoxycinnamic acid (4-Methoxycinnamic acid,p-MCA), an active rice bran phenolic acid on biotransforming bacterial enzymes and xenobiotic metabolizing enzymes in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. |
| Kinase Assay | Mechanisms of p-methoxycinnamic acid-induced increase in insulin secretion.[Pubmed: 22009371 ]Inhibitory effects of cinnamic acid and its derivatives on the diphenolase activity of mushroom ( Agaricus bisporus ) tyrosinase.[Reference: WebLink]Food Chem.,2005, 92(4):707-12.The effects of cinnamic acid and its derivatives (2-hydroxycinnamic acid, 4-hydroxycinnamic acid and 4-Methoxycinnamic acid) on the activity of mushroom tyrosinase have been studied. Horm. Metab. Res.,2011 Oct;43(11): 766-73.p-Methoxycinnamic acid (4-Methoxycinnamic acid,p-MCA) is a cinnamic acid derivative that shows various pharmacologic actions such as hepatoprotective and antihyperglycemic activities. |

4-Methoxycinnamic acid Dilution Calculator

4-Methoxycinnamic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6123 mL | 28.0615 mL | 56.123 mL | 112.246 mL | 140.3076 mL |
| 5 mM | 1.1225 mL | 5.6123 mL | 11.2246 mL | 22.4492 mL | 28.0615 mL |
| 10 mM | 0.5612 mL | 2.8062 mL | 5.6123 mL | 11.2246 mL | 14.0308 mL |
| 50 mM | 0.1122 mL | 0.5612 mL | 1.1225 mL | 2.2449 mL | 2.8062 mL |
| 100 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1225 mL | 1.4031 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Skimmianin
Catalog No.:BCN3468
CAS No.:83-95-4
- Tabernanthine
Catalog No.:BCN6957
CAS No.:83-94-3
- Riboflavine
Catalog No.:BCN2224
CAS No.:83-88-5
- Phytic acid
Catalog No.:BCN1282
CAS No.:83-86-3
- Rotenone
Catalog No.:BCN5412
CAS No.:83-79-4
- Ibogaine
Catalog No.:BCN4378
CAS No.:83-74-9
- 2-Hydroxy-1,4-naphoquinone
Catalog No.:BCN8398
CAS No.:83-72-7
- Theobromine
Catalog No.:BCN1227
CAS No.:83-67-0
- 5-Amino-1-naphthol
Catalog No.:BCC8729
CAS No.:83-55-6
- Hyodeoxycholic acid
Catalog No.:BCN1287
CAS No.:83-49-8
- Stigmasterol
Catalog No.:BCN4376
CAS No.:83-48-7
- Beta-Sitosterol
Catalog No.:BCN1015
CAS No.:83-46-5
- Methyl 3-hydroxy-4,5-dimethoxybenzoate
Catalog No.:BCN1337
CAS No.:83011-43-2
- 1,6-Dioxaspiro[4.5]decan-2-methanol
Catalog No.:BCN4371
CAS No.:83015-88-7
- Phlogacantholide B
Catalog No.:BCN7487
CAS No.:830347-16-5
- Phlogacanthoside A
Catalog No.:BCN7540
CAS No.:830347-18-7
- Ethyl gallate
Catalog No.:BCN4373
CAS No.:831-61-8
- (-)-Epicatechin-3-(3''-O-methyl) gallate
Catalog No.:BCN3062
CAS No.:83104-86-3
- (-)-Epigallocatechin-3-(3''-O-methyl) gallate
Catalog No.:BCN1336
CAS No.:83104-87-4
- 5-Hydroxymethyl-7-methoxybenzofuran
Catalog No.:BCN4372
CAS No.:831222-78-7
- 4',5-Dihydroxy-3',5',6,7-tetramethoxyflavone
Catalog No.:BCN1335
CAS No.:83133-17-9
- 3-Hydroxymethylenetanshinquinone
Catalog No.:BCN2492
CAS No.:83145-47-5
- (+)-Sophoridine
Catalog No.:BCC8360
CAS No.:83148-91-8
- Octreotide acetate
Catalog No.:BCC5643
CAS No.:83150-76-9
Natural products modulate Shigella-host-cell interaction.[Pubmed:21719574]
J Med Microbiol. 2011 Nov;60(Pt 11):1626-32.
This study focused on identifying possible new options derived from natural sources for the treatment of bacterial infections. Several natural products were investigated for their potential in modulating Shigella-host-cell interactions. The proliferation of Shigella sonnei was effectively inhibited inside HEp-2 cells in the presence of 4-Methoxycinnamic acid and propolin D. Propolin D also significantly reduced the apoptosis of infected macrophage-like U937 cells and moderately reduced the secretion of interleukin (IL)-1beta and IL-18, which probably resulted from the inhibition of invasion plasmid antigen B secretion by this compound. Further characterization showed that propolin D did not prevent escape of Shigella from phagocytic vacuoles, as evidenced by actin-based motility and by the fact that addition of chloroquine did not further reduce the number of intracellular c.f.u. The role of propolin D in modulating autophagy could not be established under the experimental conditions used. As these compounds had no direct anti-Shigella activity in vitro, it was concluded that these compounds modulated Shigella-host-cell interactions by targeting yet-to-be defined mechanisms that provide benefits to host cells.
Protective effect of p-methoxycinnamic acid, an active phenolic acid against 1,2-dimethylhydrazine-induced colon carcinogenesis: modulating biotransforming bacterial enzymes and xenobiotic metabolizing enzymes.[Pubmed:24908112]
Mol Cell Biochem. 2014 Sep;394(1-2):187-98.
Objective of the study is to evaluate the modifying potential of p-methoxycinnamic acid (p-MCA), an active rice bran phenolic acid on biotransforming bacterial enzymes and xenobiotic metabolizing enzymes in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. 48 male albino wistar rats were divided into six groups. Group1 (control) received modified pellet diet and 0.1 % carboxymethylcellulose; group2 received modified pellet diet along with p-MCA (80 mg/kg b.wt. p.o.) everyday for 16 weeks; groups 3-6 received 1,2-dimethylhydrazine (DMH) (20 mg/kg b.wt.) subcutaneous injection once a week for the first 4 weeks, while groups 4-6 received p-MCA at three different doses of 20, 40 and 80 mg/kg b.wt. p.o. everyday for 16 weeks. A significant increase in carcinogen-activating enzymes (cytochrome P450, cytochrome b5, cytochrome P4502E1, NADH-cytochrome-b5-reductase and NADPH-cytochrome-P450 reductase) with concomitant decrease in phaseII enzymes, DT-Diaphorase, glutathione S-transferase, UDP-glucuronyl-transferase and gamma glutamyltransferase were observed in group3 compared to control. DMH treatment significantly increased the activities of feacal and colonic bacterial enzymes (beta-glucosidase, beta-galactosidase, beta-glucuronidase, nitroreductase, sulphatase and mucinase). p-MCA supplementation (40 mg/kg b.wt) to carcinogen exposed rats inhibited these enzymes, which were near those of control rats. The formation of dysplastic aberrant crypt foci in the colon and the histopathological observations of the liver also supports our biochemical findings. p-MCA (40 mg/kg b.wt.) offers remarkable modulating efficacy of biotransforming bacterial and xenobiotic metabolizing enzymes in colon carcinogenesis.
Mechanisms of p-methoxycinnamic acid-induced increase in insulin secretion.[Pubmed:22009371]
Horm Metab Res. 2011 Oct;43(11):766-73.
p-Methoxycinnamic acid (p-MCA) is a cinnamic acid derivative that shows various pharmacologic actions such as hepatoprotective and antihyperglycemic activities. The present study was to elucidate the mechanisms by which p-MCA increases [Ca(2)(+)]i and insulin secretion in INS-1 cells. p-MCA (100 muM) increased [Ca(2)(+)]i in INS-1 cells. The p-MCA-induced insulin secretion and rise in [Ca(2)(+)]i were markedly inhibited in the absence of extracellular Ca(2)(+) or in the presence of an L-type Ca(2)(+) channel blocker nimodipine. These results suggested that p-MCA increased Ca(2)(+) influx via the L-type Ca(2)(+) channels. Diazoxide, an ATP-sensitive K(+) channel opener, did not alter p-MCA-induced insulin secretion, nor [Ca(2)(+)]i response. In addition, p-MCA enhanced glucose-, glibenclamide-induced insulin secretion whereas it also potentiated the increase in insulin secretion induced by arginine, and Bay K 8644, an L-type Ca(2)(+) channel agonist. Taken together, our results suggest that p-MCA stimulated insulin secretion from pancreatic beta-cells by increasing Ca(2)(+) influx via the L-type Ca(2)(+) channels, but not through the closure of ATP-sensitive K(+) channels.


