AcetoxyisovalerylalkanninCAS# 69091-17-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 69091-17-4 | SDF | Download SDF |
| PubChem ID | 155245 | Appearance | Red powder |
| Formula | C23H26O8 | M.Wt | 430.5 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
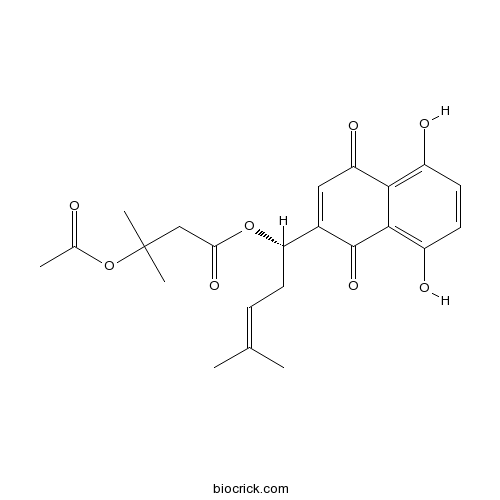
| Chemical Name | [(1S)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] 3-acetyloxy-3-methylbutanoate | ||
| SMILES | CC(=CCC(C1=CC(=O)C2=C(C=CC(=C2C1=O)O)O)OC(=O)CC(C)(C)OC(=O)C)C | ||
| Standard InChIKey | BQSAGDWOHVQNFB-SFHVURJKSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Acetoxyisovalerylalkannin may have anti-ageing activity, it can upregulate Collagen-I, elastin and involucrin syntheses in human dermal fibroblasts or keratinocytes. |
| In vitro | Anti-skin ageing activity of napthoquinones from Arnebia nobilis Reichb.f.[Pubmed: 25810219]Natural Product Research, 2015, Mar 26,30(5):574-577.The present isolation and identification of napthoquinones from roots of Arnebia nobilis Reichb.f. can lead to the discovery of new anti-skin ageing ingredient in colour cosmetics.
|
| Structure Identification | Journal of Jilin University, 2010 , 48 (2) :319-322.Chemical Constituents from Root of Arnebia euchroma(Royle)Johnst[Reference: WebLink]All the compounds were separated from the petroleum ether extracts of Arnebia euchroma root by means of column chromatography and their structures were identified by the spectral analysis and chemical evidence.
|

Acetoxyisovalerylalkannin Dilution Calculator

Acetoxyisovalerylalkannin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3229 mL | 11.6144 mL | 23.2288 mL | 46.4576 mL | 58.072 mL |
| 5 mM | 0.4646 mL | 2.3229 mL | 4.6458 mL | 9.2915 mL | 11.6144 mL |
| 10 mM | 0.2323 mL | 1.1614 mL | 2.3229 mL | 4.6458 mL | 5.8072 mL |
| 50 mM | 0.0465 mL | 0.2323 mL | 0.4646 mL | 0.9292 mL | 1.1614 mL |
| 100 mM | 0.0232 mL | 0.1161 mL | 0.2323 mL | 0.4646 mL | 0.5807 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Demanyl phosphate
Catalog No.:BCN1796
CAS No.:6909-62-2
- 2-Caren-10-ol
Catalog No.:BCN4253
CAS No.:6909-19-9
- 4'-Prenyloxyresveratrol
Catalog No.:BCN2937
CAS No.:69065-16-3
- Peonidin-3-O-glucoside chloride
Catalog No.:BCN3028
CAS No.:6906-39-4
- Delphinidin-3-O-glucoside chloride
Catalog No.:BCN3020
CAS No.:6906-38-3
- Nedocromil
Catalog No.:BCC5283
CAS No.:69049-73-6
- Hydroxytyrosol acetate
Catalog No.:BCN2963
CAS No.:69039-02-7
- Grifolin
Catalog No.:BCN7553
CAS No.:6903-07-7
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
- ZM 306416
Catalog No.:BCC3964
CAS No.:690206-97-4
- Germacrone
Catalog No.:BCN4981
CAS No.:6902-91-6
- Genipin
Catalog No.:BCN5932
CAS No.:6902-77-8
- 16-Hydroxy-8(17),13-labdadien-15,16-olid-19-oic acid
Catalog No.:BCN1378
CAS No.:691009-85-5
- Moracin C
Catalog No.:BCN4254
CAS No.:69120-06-5
- Moracin D
Catalog No.:BCN4255
CAS No.:69120-07-6
- Fiacitabine
Catalog No.:BCC1574
CAS No.:69123-90-6
- Aromadendrin 7-O-rhamnoside
Catalog No.:BCN8114
CAS No.:69135-41-7
- Malic acid
Catalog No.:BCN2699
CAS No.:6915-15-7
- Tigloylgomisin P
Catalog No.:BCN6926
CAS No.:69176-51-8
- Gomisin N
Catalog No.:BCN2271
CAS No.:69176-52-9
- (2S,3R,E)-2-Amino-4-heptadecene-1,3-diol
Catalog No.:BCN1765
CAS No.:6918-48-5
- Nesbuvir
Catalog No.:BCC1796
CAS No.:691852-58-1
- (±)-Hexanoylcarnitine chloride
Catalog No.:BCC6680
CAS No.:6920-35-0
- 2'-Hydroxy-4'-methylacetophenone
Catalog No.:BCN7751
CAS No.:6921-64-8
Anti-skin ageing activity of napthoquinones from Arnebia nobilis Reichb.f.[Pubmed:25810219]
Nat Prod Res. 2016;30(5):574-7.
The present isolation and identification of napthoquinones from roots of Arnebia nobilis Reichb.f. can lead to the discovery of new anti-skin ageing ingredient in colour cosmetics. Four compounds have been isolated and purified by rigorous column chromatography. The compounds are identified as beta, beta-dimethylacryl alkannin (AN-I), acetoxyisovaleryl alkannin (AAN-II), acetyl alkannin (AN-III) and alkannin (AN-IV) by interpretation of spectroscopic data. This study is the first to report the isolation of Acetoxyisovaleryl alkannin (AAN-II) from A. nobilis. The IC50 values of the compounds, determined in human skin cells (human dermal fibroblasts and human keratinocytes) and mouse embryonic fibroblasts (NIH3T3) varied significantly among the four alkannins. Among the four compounds, beta-acetoxyisovaleryl alkannin (AAN-II) significantly inhibited hydrogen peroxide (H2O2)-induced red blood corpuscle haemolysis and cellular senescence in human dermal fibroblasts. Collagen-I, elastin and involucrin syntheses in human dermal fibroblasts or keratinocytes were up regulated by AAN-II. These results support the potential utility of alkannins as novel anti-ageing ingredients.


