FiacitabineInhibitor of HSV DNA replication CAS# 69123-90-6 |
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- Rosiglitazone
Catalog No.:BCC2264
CAS No.:122320-73-4
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- Clofibric Acid
Catalog No.:BCC4652
CAS No.:882-09-7
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 69123-90-6 | SDF | Download SDF |
| PubChem ID | 50312 | Appearance | Powder |
| Formula | C9H11FIN3O4 | M.Wt | 371.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 382097; FIAC; FOAC | ||
| Solubility | DMSO : ≥ 37 mg/mL (99.70 mM) *"≥" means soluble, but saturation unknown. | ||
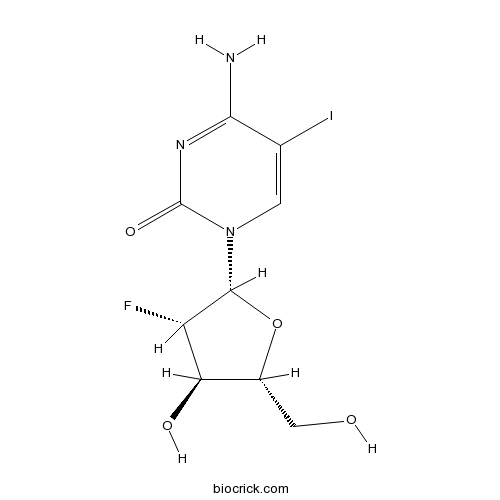
| Chemical Name | 4-amino-1-[(2R,3S,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidin-2-one | ||
| SMILES | C1=C(C(=NC(=O)N1C2C(C(C(O2)CO)O)F)N)I | ||
| Standard InChIKey | GIMSJJHKKXRFGV-BYPJNBLXSA-N | ||
| Standard InChI | InChI=1S/C9H11FIN3O4/c10-5-6(16)4(2-15)18-8(5)14-1-3(11)7(12)13-9(14)17/h1,4-6,8,15-16H,2H2,(H2,12,13,17)/t4-,5+,6-,8-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fiacitabine(NSC 382097; FIAC; FOAC) is a selective inhibitior of DNA replication of herpes simplex virus(HSV) with IC50 values of 2.5 nM and 12.6 nM for HSV1 and HSV2, respectively.
IC50 value: 2.5/12.6 nM (HSV1/2) [2]
Target: HSV
FIAC suppressed by 90% the replication of various strains of herpes simplex virus types 1 and 2 at concentrations of 0.0025 to 0.0126 microM. Cytotoxicity was minimal, as determined by trypan blue dye exclusion with norman Vero, WI-38, and NC-37 cell proliferation; the 50% inhibitory dose was 4 to 10 microM in a 4-day assay. FIAC was active at much lower concentrations than arabinosylcytosine, iododeoxyuridine, and arabinosyladenine. It was slightly more active against herpes simplex virus type 1 than acycloquanosine and slightly more toxic to normal cells. FIAC was about 8,000 times more active against the replication of wild-type herpes simplex virus type 1 than against a mutant strain lacking the expression of virus-specified thymidine kinase [2]. References: | |||||

Fiacitabine Dilution Calculator

Fiacitabine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6946 mL | 13.4731 mL | 26.9462 mL | 53.8924 mL | 67.3655 mL |
| 5 mM | 0.5389 mL | 2.6946 mL | 5.3892 mL | 10.7785 mL | 13.4731 mL |
| 10 mM | 0.2695 mL | 1.3473 mL | 2.6946 mL | 5.3892 mL | 6.7365 mL |
| 50 mM | 0.0539 mL | 0.2695 mL | 0.5389 mL | 1.0778 mL | 1.3473 mL |
| 100 mM | 0.0269 mL | 0.1347 mL | 0.2695 mL | 0.5389 mL | 0.6737 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fiacitabine(NSC 382097; FIAC; FOAC) is a selective inhibitior of DNA replication of herpes simplex virus(HSV) with IC50 values of 2.5 nM and 12.6 nM for HSV1 and HSV2, respectively.
- Moracin D
Catalog No.:BCN4255
CAS No.:69120-07-6
- Moracin C
Catalog No.:BCN4254
CAS No.:69120-06-5
- 16-Hydroxy-8(17),13-labdadien-15,16-olid-19-oic acid
Catalog No.:BCN1378
CAS No.:691009-85-5
- Acetoxyisovalerylalkannin
Catalog No.:BCN3007
CAS No.:69091-17-4
- Demanyl phosphate
Catalog No.:BCN1796
CAS No.:6909-62-2
- 2-Caren-10-ol
Catalog No.:BCN4253
CAS No.:6909-19-9
- 4'-Prenyloxyresveratrol
Catalog No.:BCN2937
CAS No.:69065-16-3
- Peonidin-3-O-glucoside chloride
Catalog No.:BCN3028
CAS No.:6906-39-4
- Delphinidin-3-O-glucoside chloride
Catalog No.:BCN3020
CAS No.:6906-38-3
- Nedocromil
Catalog No.:BCC5283
CAS No.:69049-73-6
- Hydroxytyrosol acetate
Catalog No.:BCN2963
CAS No.:69039-02-7
- Grifolin
Catalog No.:BCN7553
CAS No.:6903-07-7
- Aromadendrin 7-O-rhamnoside
Catalog No.:BCN8114
CAS No.:69135-41-7
- Malic acid
Catalog No.:BCN2699
CAS No.:6915-15-7
- Tigloylgomisin P
Catalog No.:BCN6926
CAS No.:69176-51-8
- Gomisin N
Catalog No.:BCN2271
CAS No.:69176-52-9
- (2S,3R,E)-2-Amino-4-heptadecene-1,3-diol
Catalog No.:BCN1765
CAS No.:6918-48-5
- Nesbuvir
Catalog No.:BCC1796
CAS No.:691852-58-1
- (±)-Hexanoylcarnitine chloride
Catalog No.:BCC6680
CAS No.:6920-35-0
- 2'-Hydroxy-4'-methylacetophenone
Catalog No.:BCN7751
CAS No.:6921-64-8
- Osthol hydrate
Catalog No.:BCN1377
CAS No.:69219-24-5
- Pinoresinol 4-O-beta-D-glucopyranoside
Catalog No.:BCN1376
CAS No.:69251-96-3
- 1,2,3-Tri-O-methyl-7,8-methyleneflavellagic acid
Catalog No.:BCN7205
CAS No.:69251-99-6
- N-Benzylcinchonidinium chloride
Catalog No.:BCC9094
CAS No.:69257-04-1
Spectrum of activity and mechanisms of resistance of various nucleoside derivatives against gammaherpesviruses.[Pubmed:25267682]
Antimicrob Agents Chemother. 2014 Dec;58(12):7312-23.
The susceptibilities of gammaherpesviruses, including Epstein-Barr virus (EBV), Kaposi's sarcoma-associated herpesvirus (KSHV), and animal rhadinoviruses, to various nucleoside analogs was investigated in this work. Besides examining the antiviral activities and modes of action of antivirals currently marketed for the treatment of alpha- and/or betaherpesvirus infections (including acyclovir, ganciclovir, penciclovir, foscarnet, and brivudin), we also investigated the structure-activity relationship of various 5-substituted uridine and cytidine molecules. The antiviral efficacy of nucleoside derivatives bearing substitutions at the 5 position was decreased if the bromovinyl was replaced by chlorovinyl. 1-beta-D-Arabinofuranosyl-(E)-5-(2-bromovinyl)uracil (BVaraU), a nucleoside with an arabinose configuration of the sugar ring, exhibited no inhibitory effect against rhadinoviruses but was active against EBV. On the other hand, the fluoroarabinose cytidine analog 2'-fluoro-5-iodo-aracytosine (FIAC) showed high selectivity indices against gammaherpesviruses that were comparable to those of brivudin. Additionally, we selected brivudin- and acyclovir-resistant rhadinoviruses in vitro and characterized them by phenotypic and genotypic (i.e., sequencing of the viral thymidine kinase, protein kinase, and DNA polymerase) analysis. Here, we reveal key amino acids in these enzymes that play an important role in substrate recognition. Our data on drug susceptibility profiles of the different animal gammaherpesvirus mutants highlighted cross-resistance patterns and indicated that pyrimidine nucleoside derivatives are phosphorylated by the viral thymidine kinase and purine nucleosides are preferentially activated by the gammaherpesvirus protein kinase.
Current pharmacological approaches to the therapy of varicella zoster virus infections: a guide to treatment.[Pubmed:10188760]
Drugs. 1999 Feb;57(2):187-206.
Varicella zoster virus (VZV), a member of the herpesvirus family, is responsible for both primary (varicella, chickenpox) as well as reactivation (zoster, shingles) infections. In immunocompetent patients, the course of varicella is generally benign. For varicella zoster, post-herpetic neuralgia is the most common complication. In immunocompromised patients (particularly those with AIDS), transplant recipients and cancer patients, VZV infections can be life-threatening. For these patients and also for immunocompetent patients at risk such as pregnant women or premature infants, the current treatment of choice is based on either intravenous or oral aciclovir (acyclovir). The low oral bioavailability of aciclovir, as well as the emergence of drug-resistant virus strains, have stimulated efforts towards the development of new compounds for the treatment of individuals with VZV infections. Among these new compounds, penciclovir, its oral prodrug form famciclovir and the oral pro-drug form of aciclovir (valaciclovir), rank among the most promising. As with aciclovir itself, all of these drugs are dependent on the virus-encoded thymidine kinase (TK) for their intracellular activation (phosphorylation), and, upon conversion to their triphosphate form, they act as inhibitors/alternative substrate of the viral DNA polymerase. Therefore, cross-resistance to these drugs may be expected for those virus mutants that are TK-deficient and thus resistant to aciclovir. Other classes of nucleoside analogues dependent for their phosphorylation on the viral TK that have been pursued for the treatment of VZV infections include sorivudine, brivudine, fialuridine, Fiacitabine and netivudine. Among oxetanocins, which are partially dependent on viral TK, lobucavir is now under clinical evaluation. Foscarnet, which does not require any previous metabolism to interact with the viral DNA polymerase, is used in the clinic when TK-deficient VZV mutants emerge during aciclovir treatment. TK-deficient mutants are also sensitive to the acyclic nucleoside phosphonates (i.e. [s]-1-[3-hydroxy-2-phosphonylmethoxypropyl]cytosine; HPMPC); these agents do not depend on the virus-encoded TK for their phosphorylation but depend on cellular enzymes for conversion to their diphosphoryl derivatives which then inhibit viral DNA synthesis. Vaccination for VZV has now come of age. It is recommended for healthy children, patients with leukaemia, and patients receiving immunosuppressive therapy or those with chronic diseases. The protection induced by the vaccine seems, to some extent, to include zoster and associated neuralgia. Passive immuniatin based on specific immunoglobulins does not effectively prevent VZV infection and is therefore restricted to high risk individuals (i.e. immunocompromised children and pregnant women).
Evaluation of F-18-labeled 5-iodocytidine (18F-FIAC) as a new potential positron emission tomography probe for herpes simplex virus type 1 thymidine kinase imaging.[Pubmed:21982570]
Nucl Med Biol. 2011 Oct;38(7):987-95.
OBJECTIVE: Herpes simplex virus type 1 thymidine kinase (HSV1-tk) gene in combination with radiolabeled nucleoside substrates is the most widely used reporter system. This study characterized 1-(2'-deoxy-2'-[(18)F]fluoro-beta-D-arabinofuranosyl)-5-iodocytosine ((18)F-FIAC) as a new potential positron emission tomography (PET) probe for HSV1-tk gene imaging and compared it with 2'-deoxy-2'-[(18)F]fluoro-5-iodo-1-beta-D-arabinofuranosyluracil ((18)F-FIAU) and 2'-deoxy-2'-[(18)F]fluoro-5-ethyl-1-beta-D-arabinofuranosyluracil((18)F-FEAU) (thymidine analogues) in an NG4TL4-WT/STK sarcoma-bearing mouse model. METHODS: A cellular uptake assay, biodistribution study, radioactive metabolites assay and microPET imaging of NG4TL4-WT/STK tumor-bearing mice post administration of (18)F-FIAC, (18)F-FIAU and (18)F-FEAU were conducted to characterize the biological properties of these tracers. RESULTS: Highly specific uptake of (18)F-FIAC, (18)F-FIAU and (18)F-FEAU in tk-transfected [tk(+)] cells was observed. The tk(+)-to-tk(-) cellular uptake ratio after a 2-h incubation was 66.6+/-25.1, 76.3+/-18.2 and 247.2+/-37.2, respectively. In biodistribution studies, (18)F-FIAC showed significant tk(+) tumor specificity (12.6; expressed as the tk(+)-to-tk(-) tumor uptake ratio at 2 h postinjection) comparable with (18)F-FIAU (15.8) but lower than (18)F-FEAU (48.0). The results of microPET imaging also revealed the highly specific accumulation of these three radioprobes in the NG4TL4-tk(+) tumor. CONCLUSION: Our findings suggested that the cytidine analogue (18)F-FIAC is a new potential PET probe for the imaging of HSV1-tk gene expression. (18)F-FIAC may be regarded as the prodrug of (18)F-FIAU in vivo.
Radiosynthesis of F-18 labeled cytidine analog 2'-fluoro-5-iodo-l-beta-d-arabinofuranosylcytosine ([(18)F]FIAC).[Pubmed:19324560]
Appl Radiat Isot. 2009 Jul-Aug;67(7-8):1362-5.
We reported the synthesis of 2'-deoxy-2'-[(18)F]fluoro-5-iodo-1-beta-d-arabinofuranosyl-5-iodo-cytosine ([(18)F]FIAC) with 15-20% radiochemical yield (decay corrected) in 3.5h. 2-deoxy-2-[(18)F]fluoro-1,3,5-tri-O-benzoyl-alpha-d-arabinofuranose was prepared following literature procedures with some modifications (yield>70%). The (18)F-fluorosugar was converted to 1-bromo-(18)F-fluorosugar, and then coupled with 5-iodocytocine silyl ether. A mixture of acetonitrile (ACN) and 1,2-dichloroethane (DCE) were employed to achieve optimum radiochemical yield and acceptable beta-anomer selectivity (alpha/beta=1/3). After hydrolyzed with sodium methoxide, the crude product was purified using HPLC to afford the beta-[(18)F]FIAC with high radiochemical purity (>or=98%).
Therapeutic gene expression in transduced mesenchymal stem cells can be monitored using a reporter gene.[Pubmed:22796395]
Nucl Med Biol. 2012 Nov;39(8):1243-50.
AIM: We constructed a recombinant adenovirus construct Ad5-sr39tk-IRES-VEGF(165) (Ad5-SIV) that contained a mutant herpes viral thymidine kinase reporter gene (HSV1-sr39tk) and the human vascular endothelial growth factor 165 (VEGF(165)) gene for noninvasive imaging of gene expression. The recombinant adenovirus Ad5-SIV was transfected into rat bone marrow-derived mesenchymal stem cells (MSCs), and we measured the expression of HSV1-sr39tk and VEGF(165) to evaluate the feasibility of monitoring VEGF(165) expression using reporter gene expression. METHODS: The MSCs were infected with Ad5-SIV at various levels of infection (MOI), ranging from 0 to 100 infectious units per cell (IU/cell). The mRNA and protein expression levels of the reporter and therapeutic genes were determined using real-time RT-PCR, Western blot, ELISA and immunofluorescence. The HSV1-sr39tk expression in the MSCs was also detected in vitro using a cellular uptake study of the reporter probe (131)I-FIAU. Gene expression was also evaluated in vivo by micro-Positron Emission Tomography/Computed Tomography (micro-PET/CT) imaging 1day after injecting Ad5-SIV-tranfected MSCs into the left foreleg of the rat. The right foreleg was injected with non-transfected MSCs and served as an internal control. RESULTS: The real-time RT-PCR results demonstrated a good correlation between the expression levels of HSV1-sr39tk mRNA and VEGF(165) mRNA (R(2)=0.93, P<0.05). The cellular uptake of (131)I-FIAU increased with increasing viral titers (R(2)=0.89; P<0.05), and in the group that received an MOI of 100, a peak value of 30.15%+/-1.11% was found at 3 hours of incubation. The uptake rates increased rapidly between 30 and 150 minutes and reached a plateau after 150 minutes. The uptake rates of (131)I-FIAU by the Ad5-SIV-infected cells were significantly higher than by the Ad5-EGFP-infected cells for all time points (t=18.43-54.83, P<0.05). Moreover, the rate of VEGF(165) protein secretion was highly correlated with the uptake rate of (131)I-FIAU (R(2)=0.84, P<0.05). The radioactivity on the micro-PET/CT images was significantly higher in the left foreleg (which received the transfected MSCs) compared with the control foreleg. CONCLUSIONS: These results suggest that radionuclide reporter gene imaging may be used to monitor gene expression in vivo.


