Albanol BCAS# 87084-99-9 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 87084-99-9 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
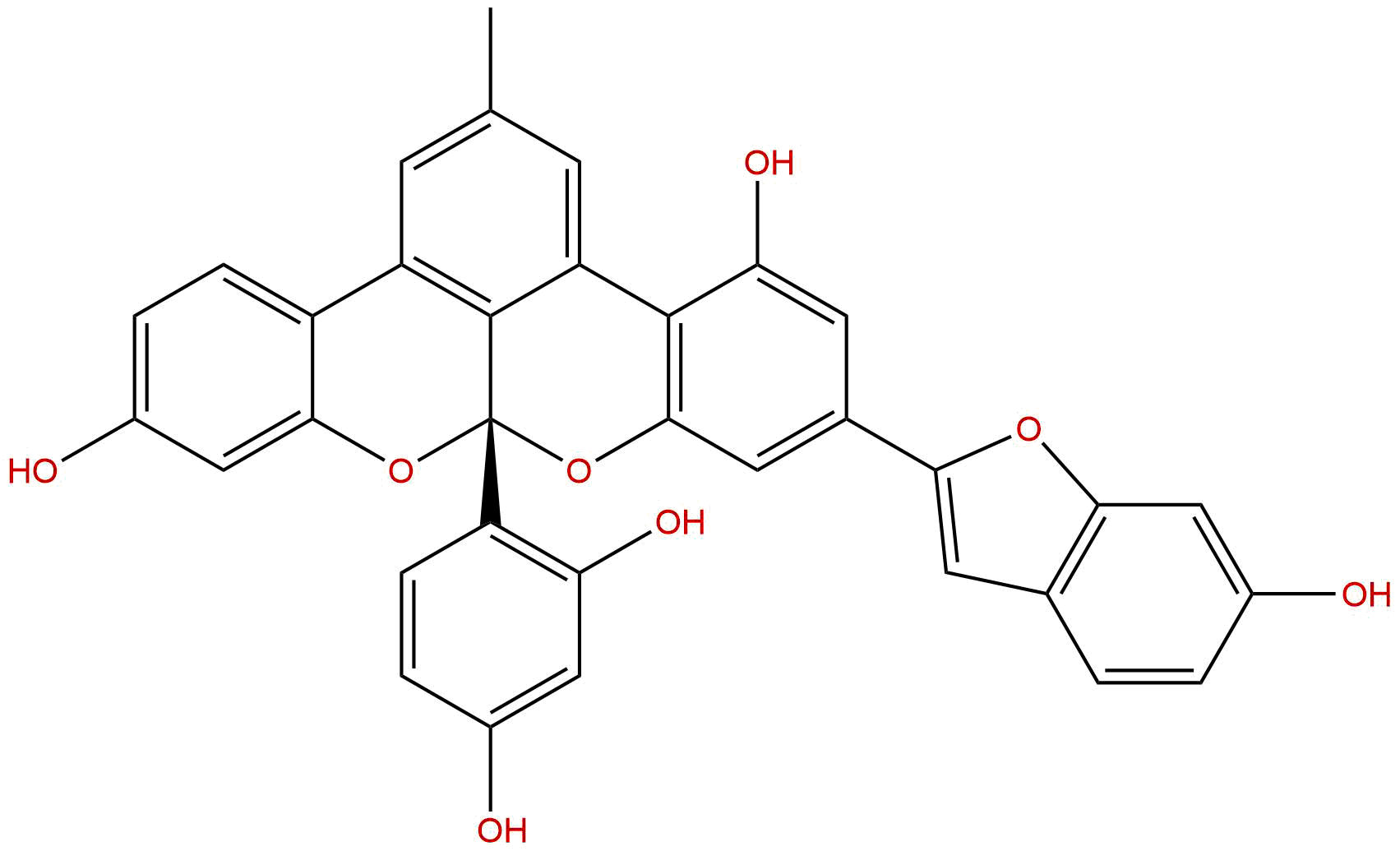
| Formula | C34H22O8 | M.Wt | 558.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Albanol B Dilution Calculator

Albanol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7904 mL | 8.9519 mL | 17.9038 mL | 35.8076 mL | 44.7596 mL |
| 5 mM | 0.3581 mL | 1.7904 mL | 3.5808 mL | 7.1615 mL | 8.9519 mL |
| 10 mM | 0.179 mL | 0.8952 mL | 1.7904 mL | 3.5808 mL | 4.476 mL |
| 50 mM | 0.0358 mL | 0.179 mL | 0.3581 mL | 0.7162 mL | 0.8952 mL |
| 100 mM | 0.0179 mL | 0.0895 mL | 0.179 mL | 0.3581 mL | 0.4476 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- t-OMe-Byakangelicin
Catalog No.:BCX1236
CAS No.:89560-97-4
- Hedysarimcoumestan B
Catalog No.:BCX1235
CAS No.:899436-04-5
- 2-(3,4-dihydroxyphenyl)-7-(β-D-glucopyranosyloxy)-8-hydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX1234
CAS No.:925701-05-9
- Sikokianin E
Catalog No.:BCX1233
CAS No.:2253791-96-5
- Isocoreopsin
Catalog No.:BCX1232
CAS No.:30382-18-4
- Dihydrobaicalin
Catalog No.:BCX1231
CAS No.:56226-98-3
- Segetalin C
Catalog No.:BCX1230
CAS No.:177602-12-9
- 2,7-dihydroxy-4, 6-dimethoxy phenanthrene
Catalog No.:BCX1229
CAS No.:108352-70-1
- Argentinogenin
Catalog No.:BCX1228
CAS No.:4236-48-0
- β-Obscurin
Catalog No.:BCX1227
CAS No.:467-79-8
- Cnidimol B
Catalog No.:BCX1226
CAS No.:103629-81-8
- Erythro-guaiacylglycerol-β-O-4'-sinapyl ether
Catalog No.:BCX1225
CAS No.:905726-70-7
- Ekersenin
Catalog No.:BCX1238
CAS No.:53091-74-0
- Oxypeucedanin methanolate
Catalog No.:BCX1239
CAS No.:52939-12-5
- 1-O-gentiobiosyl-3,7-dimethoxy-8-hydroxyxanthone
Catalog No.:BCX1240
CAS No.:487040-33-5
- Chikusaikoside II
Catalog No.:BCX1241
CAS No.:166338-14-3
- Damnacanthol
Catalog No.:BCX1242
CAS No.:477-83-8
- Aconicarchamine B
Catalog No.:BCX1243
CAS No.:1275535-67-5
- Hypolaetin 7-O-glucoside
Catalog No.:BCX1244
CAS No.:32455-43-9
- Hypoletin-7-O-β-D-xylopyranoside
Catalog No.:BCX1245
CAS No.:126771-28-6
- Carmichasine B
Catalog No.:BCX1246
CAS No.:2245700-60-9
- trans-Ferulic acid-4-β-glucoside
Catalog No.:BCX1247
CAS No.:117405-51-3
- 16β-Hydroperoxyalisol B 23-acetate
Catalog No.:BCX1248
CAS No.:2221029-54-3
- 11(α)-Hydroxynepasaikosaponin k
Catalog No.:BCX1249
CAS No.:1152168-63-2
Albanol B from Mulberries Exerts Anti-Cancer Effect through Mitochondria ROS Production in Lung Cancer Cells and Suppresses In Vivo Tumor Growth.[Pubmed:33327489]
Int J Mol Sci. 2020 Dec 14;21(24):9502.
Albanol B (ABN-B), an arylbenzofuran derivative isolated from mulberries, has been shown to have anti-Alzheimer's disease, anti-bacterial and antioxidant activities. The aim of this study was to investigate the anti-cancer effect of this compound against lung cancer cells. The results show that ABN-B inhibited the proliferation of four human lung cancer cell lines (A549, BZR, H1975, and H226) and induced apoptosis, based on the cleavage of caspase-7 and PARP (poly (ADP-ribose) polymerase), as well as the downregulation of Bcl-2. ABN-B also induced cell cycle arrest at G(2)/M by down-regulating the expression of CKD1 (cyclin-dependent kinase 1) and cyclin B1, but up-regulating p21 (cyclin-dependent kinase inhibitor 1) expression. Notably, ABN-B increased the production of mitochondrial reactive oxygen species (ROS); however, treatment with mito-TEMPO (a specific mitochondrial antioxidant) blocked ABN-B-induced cell cycle arrest at G(2)/M and apoptosis, as well as the up-regulation of p21 and down-regulation of CDK1 and cyclin B1 induced by ABN-B. At the molecular level, ABN-B-induced mitochondrial ROS production increased the phosphorylation levels of AKT (protein kinase B) and ERK1/2 (extracellular signal-regulated kinase 1/2), while the inhibition of these kinases blocked the ABN-B-induced up-regulation of p21 and down-regulation of CDK1 and cyclin B1. Moreover, ABN-B significantly suppressed tumor growth in Ex-3LL (Lewis lung carcinoma) tumor-bearing mice. Taken together, these results suggest that ABN-B can exert an anti-cancer effect by inducing apoptosis and cell cycle arrest at G(2)/M through mitochondrial ROS production in lung cancer cells.
Novel Diels-Alder Type Adducts from Morus alba Root Bark Targeting Human Monoamine Oxidase and Dopaminergic Receptors for the Management of Neurodegenerative Diseases.[Pubmed:31835621]
Int J Mol Sci. 2019 Dec 10;20(24):6232.
In this study, we delineate the human monoamine oxidase (hMAO) inhibitory potential of natural Diels-Alder type adducts, mulberrofuran G (1), kuwanon G (2), and Albanol B (3), from Morus alba root bark to characterize their role in Parkinson's disease (PD) and depression, focusing on their ability to modulate dopaminergic receptors (D(1)R, D(2L)R, D(3)R, and D(4)R). In hMAO-A inhibition, 1-3 showed mild effects (50% inhibitory concentration (IC(50)): 54‒114 muM). However, 1 displayed moderate inhibition of the hMAO-B isozyme (IC(50): 18.14 +/- 1.06 muM) followed by mild inhibition by 2 (IC(50): 57.71 +/- 2.12 muM) and 3 (IC(50): 90.59 +/- 1.72 muM). Our kinetic study characterized the inhibition mode, and the in silico docking predicted that the moderate inhibitor 1 would have the lowest binding energy. Similarly, cell-based G protein-coupled receptors (GPCR) functional assays in vector-transfected cells expressing dopamine (DA) receptors characterized 1-3 as D(1)R/D(2L)R antagonists and D(3)R/D(4)R agonists. The half-maximum effective concentration (EC(50)) of 1-3 on DA D(3)R/D(4)R was 15.13/17.19, 20.18/21.05, and 12.63/‒ microM, respectively. Similarly, 1-3 inhibited 50% of the DA response on D(1)R/D(2L)R by 6.13/2.41, 16.48/31.22, and 7.16/18.42 microM, respectively. A computational study revealed low binding energy for the test ligands. Interactions with residues Asp110, Val111, Tyr365, and Phe345 at the D(3)R receptor and Asp115 and His414 at the D(4)R receptor explain the high agonist effect. Likewise, Asp187 at D(1)R and Asp114 at D(2L)R play a crucial role in the antagonist effects of the ligand binding. Our overall results depict 1-3 from M. alba root bark as good inhibitors of hMAO and potent modulators of DA function as D(1)R/D(2L)R antagonists and D(3)R/D(4)R agonists. These active constituents in M. alba deserve in-depth study for their potential to manage neurodegenerative disorders (NDs), particularly PD and psychosis.
Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra.[Pubmed:31288489]
Molecules. 2019 Jul 8;24(13):2497.
Black mulberry is a widely acknowledged ancient traditional medicine. Its extract and constituents have been reported to exert various bioactivities including antimicrobial, hypotensive, analgesic etc. effects. While black mulberry preparations are also used as antispasmodic agents in folk medicine, no related studies are available on its isolated constituents. Through an extensive chromatographic purification, seven phenolic compounds were isolated from the methanol extract of Morus nigra root bark, including morusin (1), kuwanon U (2), kuwanon E (3), moracin P (4), moracin O (5), albanol A (6), and Albanol B (7). A complete NMR signal assignment of moracin P and O was achieved, and related literature errors confusing the identity of moracin derivatives are hereby clarified. Compounds 2, 5 and 7 were identified as strong antispasmodic agents on isolated rat ileum and tracheal smooth muscles, while compound 3, a methoxy derivative of 2, was inactive. Moracin O (5) inhibited the ileal and tracheal smooth muscle contractions with E(max) values of 85% and 302 mg, respectively. Those actions were superior as compared with papaverine. Our findings demonstrate that prenylated arylbenzofurans, geranylated flavonoids and Diels-Alder adducts from Morus nigra are valuable antispasmodic agents. Compounds 2, 5 and 7 are suggested as marker compounds for quality control of antispasmodic mulberry preparations. Moracin O (5) is a new lead compound for related drug development initiatives.
Anti-inflammatory effects of mulberry (Morus alba L.) root bark and its active compounds.[Pubmed:30470128]
Nat Prod Res. 2020 Jun;34(12):1786-1790.
Mulberry (Morus alba L.) root bark (MRB) was extracted using methanol and the extracts were subjected to tests of anti-inflammatory effects. The ethyl acetate fraction demonstrated the best anti-inflammatory effects. Purified compounds, sanggenon B, Albanol B and sanggenon D, showed inhibitory effects on NO production in LPS-stimulated RAW264.7 cells and Albanol B demonstrated the best anti-inflammatory effects. Regarding the underlying molecular mechanisms, further investigations showed that treatments with Albanol B reduced production of pro-inflammatory cytokines and decreased expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). These results would contribute to development of novel anti-inflammatory drugs from MRB.
Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and alpha-glucosidase.[Pubmed:30103164]
Phytochemistry. 2018 Nov;155:114-125.
As part of our continuing research to obtain pharmacologically active compounds from Morus alba L. (Moraceae), four Diels-Alder type adducts (DAs) [morusalbins A-D], one isoprenylated flavonoid [albanin T], together with twenty-one known phenolic compounds were isolated from its root bark. The chemical structures were established using NMR, MS, and ECD spectra. The DAs including morusalbins A-D, albasin B, macrourin G, yunanensin A, mulberrofuran G and K, and Albanol B exhibited strong inhibitory activities against both protein tyrosine phosphatase 1B (PTP1B) (IC(50), 1.90-9.67 muM) and alpha-glucosidase (IC(50), 2.29-5.91 muM). In the kinetic study, morusalbin D, albasin B, and macrourin G showed noncompetitive PTP1B inhibition, with K(i) values of 0.33, 1.00, and 1.09 muM, respectively. In contrast, these DAs together with yunanensin A produced competitive inhibition of alpha-glucosidase, with K(i) values of 0.64, 0.42, 2.42, and 1.19 muM, respectively. Furthermore, molecular docking studies revealed that these active DAs have high affinity and tight binding capacity towards the active site of PTP1B and alpha-glucosidase.
Structure(-)Activity Relationship of the Tyrosinase Inhibitors Kuwanon G, Mulberrofuran G, and Albanol B from Morus Species: A Kinetics and Molecular Docking Study.[Pubmed:29891812]
Molecules. 2018 Jun 11;23(6):1413.
Kuwanon G (KG) and benzofuran flavonoids such as mulberrofuran G (MG) and Albanol B (AB) isolated from Morus sp. are reported to exhibit anti-Alzheimer's disease, anti-inflammatory, fungicidal, anti-cancer, anti-bacterial, and anti-tyrosinase properties. We investigated the inhibition of mono- and diphenolase activity of mushroom tyrosinase by KG, MG, and AB. KG and MG displayed acceptable inhibition activity compared to kojic acid. AB did not show any activity up to 350 microM. MG displayed six-fold higher inhibition of l-tyrosine oxidation (IC(50) = 6.35 +/- 0.45 microM) compared to kojic acid (IC(50) = 36.0 microM). Kinetic studies revealed that KG and MG inhibited monophenolase activity of tyrosinase in a competitive manner. Docking simulations of KG and MG demonstrated favorable binding energies with amino acid residues of the active sites of tyrosinase. Our investigation of the structure-activity relationship of the fused benzofuran flavonoids (MG vs. AB) implicated the methyl cyclohexene ring moiety in tyrosinase inhibition. The enzyme substrate and relative structural analyses demonstrated that KG and MG from Morus sp. could be useful natural tyrosinase inhibitors in foods or cosmetics.
Protein Tyrosine Phosphatase 1B Inhibition and Glucose Uptake Potentials of Mulberrofuran G, Albanol B, and Kuwanon G from Root Bark of Morus alba L. in Insulin-Resistant HepG2 Cells: An In Vitro and In Silico Study.[Pubmed:29786669]
Int J Mol Sci. 2018 May 22;19(5):1542.
Type II diabetes mellitus (T2DM) is the most common form of diabetes and has become a major health problem across the world. The root bark of Morus alba L. is widely used in Traditional Chinese Medicine for treatment and management of diabetes. The aim of the present study was to evaluate the enzyme inhibitory potentials of three principle components, mulberrofuran G (1), Albanol B (2), and kuwanon G (3) in M. alba root bark against diabetes, establish their enzyme kinetics, carry out a molecular docking simulation, and demonstrate the glucose uptake activity in insulin-resistant HepG2 cells. Compounds 1(-)3 showed potent mixed-type enzyme inhibition against protein tyrosine phosphatase 1B (PTP1B) and alpha-glucosidase. In particular, molecular docking simulations of 1(-)3 demonstrated negative binding energies in both enzymes. Moreover, 1(-)3 were non-toxic up to 5 microM concentration in HepG2 cells and enhanced glucose uptake significantly and decreased PTP1B expression in a dose-dependent manner in insulin-resistant HepG2 cells. Our overall results depict 1(-)3 from M. alba root bark as dual inhibitors of PTP1B and alpha-glucosidase enzymes, as well as insulin sensitizers. These active constituents in M. alba may potentially be utilized as an effective treatment for T2DM.
Anti-Alzheimer's disease activity of compounds from the root bark of Morus alba L.[Pubmed:28093699]
Arch Pharm Res. 2017 Mar;40(3):338-349.
The inhibition of acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) plays important roles in prevention and treatment of Alzheimer's disease (AD). Among the individual parts of Morus alba L. including root bark, branches, leaves, and fruits, the root bark showed the most potent enzyme inhibitory activities. Therefore, the aim of this study was to evaluate the anti-AD activity of the M. alba root bark and its isolate compounds, including mulberrofuran G (1), Albanol B (2), and kuwanon G (3) via inhibition of AChE, BChE, and BACE1. Compounds 1 and 2 showed strong AChE- and BChE-inhibitory activities; 1-3 showed significant BACE1 inhibitory activity. Based on the kinetic study with AChE and BChE, 2 and 3 showed noncompetitive-type inhibition; 1 showed mixed-type inhibition. Moreover, 1-3 showed mixed-type inhibition against BACE1. The molecular docking simulations of 1-3 demonstrated negative binding energies, indicating a high affinity to AChE and BACE1. The hydroxyl group of 1-3 formed hydrogen bond with the amino acid residues located at AChE and BACE1. Consequently, these results indicate that the root bark of M. alba and its active compounds might be promising candidates for preventive and therapeutic agents for AD.
[Study of anti-oxidant phenolic compounds from stem barks of Morus yunanensis].[Pubmed:18837317]
Zhongguo Zhong Yao Za Zhi. 2008 Jul;33(13):1569-72.
OBJECTIVE: To study anti-oxidant phenolic compounds from stem barks of Morus yunanensis. METHOD: Isolation and purification were carried out by silica gel, Sephadex LH -20 and RP - 18 column chromatography. The chemical structures of constituents were elucidated on the basis of physicochemical properties and spectral data. RESULT: Eight phenolic compounds were isolated and identified as: mulberrofuran E (1), mulberrofuran K (2), Albanol B (3), kuwanon H (4), resveratrol (5), moracin M (6), morachalcone A (7), 3', 5', 2, 4-tetrahydroxy-4-(3-methyl-1-butenyl) stilbene (8). CONCLUSION: All the compounds were isolated from the plant for the first time, and compounds 2, 3, 6, 8 showed potent anti-oxidation activities.
Five new diels-alder type adducts from the stem and root bark of Morus mongolica.[Pubmed:16450296]
Planta Med. 2006 Jan;72(1):52-9.
Five new Diels-Alder type adducts, mongolicins A - E, and nine known compounds, mongolicin F, chalcomoracin, mulberrofuran T, mulberrofuran G, mulberrofuran F, Albanol B, kuwanon O, mulberrofuran H and kuwanon H, were isolated from the stem and root bark of Morus mongolica. Their structures were determined by spectroscopic analysis and chiroptical methods. Among them, compounds and showed potent anti-inflammatory activities (inhibition of release of beta-glucuronidase from rat PMNs induced by PAF) with inhibitory ratios of 80.4% (P<0.05) and 77.0% (P<0.001) at a concentration of 10(-5) mol L-1. Compounds and were active as antioxidatives (inhibition of liver microsomal lipid peroxidation induced by Fe2+-Cys system) with inhibitory ratios of 83.6% (P<0.05), 86.0% (P<0.05), and 63.0% (P<0.05) at a concentration of 10(-5) mol L-1.
Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai.[Pubmed:15636183]
Phytomedicine. 2004 Nov;11(7-8):666-72.
Antimicrobial activity of the 18 prenylated flavonoids, which were purified from five different medicinal plants, was evaluated by determination of MIC using the broth microdilution methods against four bacterial and two fungal microorganisms (Candida albicans, Saccaromyces cerevisiae, Escherichia coli, Salmonella typhimurium, Staphylococcus epidermis and S. aureus). Papyriflavonol A, kuraridin, sophoraflavanone D and sophoraisoflavanone A exhibited a good antifungal activity with strong antibacterial activity. Kuwanon C, mulberrofuran G, Albanol B, kenusanone A and sophoraflavanone G showed strong antibacterial activity with 5-30 microg/ml of MICs. Morusin, sanggenon B and D, kazinol B, kurarinone, kenusanone C and isosophoranone were effective to only gram positive bacteria, and broussochalcone A was effective to C. albicans. IC50 values of papyriflavonol A, kuraridin, sophoraflavanone D, sophoraisoflavanone A and broussochalcone A in HepG2 cells were 20.9, 37.8, 39.1, 22.1, and 22.0 microg/ml, respectively. These results support the use of prenylated flavonoids in Asian traditional medicine to treat microbial infection and indicate a high potential for prenylated flavonoids as antimicrobial agents as well as anti-inflammatory agents.


