Oxypeucedanin methanolateCAS# 52939-12-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 52939-12-5 | SDF | Download SDF |
| PubChem ID | 15945061.0 | Appearance | Powder |
| Formula | C17H18O6 | M.Wt | 318.32 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
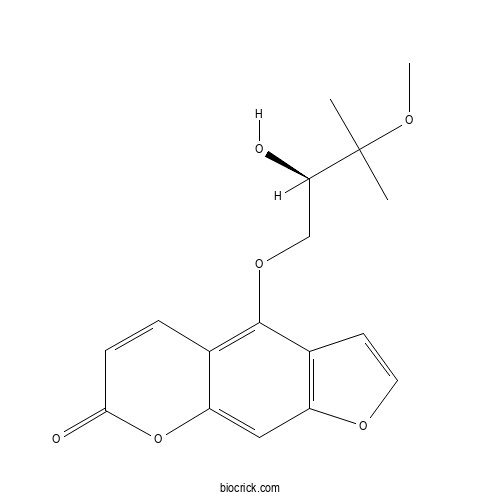
| Chemical Name | 4-[(2R)-2-hydroxy-3-methoxy-3-methylbutoxy]furo[3,2-g]chromen-7-one | ||
| SMILES | CC(C)(C(COC1=C2C=CC(=O)OC2=CC3=C1C=CO3)O)OC | ||
| Standard InChIKey | UHENVVIVPZCJOA-CQSZACIVSA-N | ||
| Standard InChI | InChI=1S/C17H18O6/c1-17(2,20-3)14(18)9-22-16-10-4-5-15(19)23-13(10)8-12-11(16)6-7-21-12/h4-8,14,18H,9H2,1-3H3/t14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Oxypeucedanin methanolate Dilution Calculator

Oxypeucedanin methanolate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1415 mL | 15.7075 mL | 31.4149 mL | 62.8299 mL | 78.5373 mL |
| 5 mM | 0.6283 mL | 3.1415 mL | 6.283 mL | 12.566 mL | 15.7075 mL |
| 10 mM | 0.3141 mL | 1.5707 mL | 3.1415 mL | 6.283 mL | 7.8537 mL |
| 50 mM | 0.0628 mL | 0.3141 mL | 0.6283 mL | 1.2566 mL | 1.5707 mL |
| 100 mM | 0.0314 mL | 0.1571 mL | 0.3141 mL | 0.6283 mL | 0.7854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ekersenin
Catalog No.:BCX1238
CAS No.:53091-74-0
- Albanol B
Catalog No.:BCX1237
CAS No.:87084-99-9
- t-OMe-Byakangelicin
Catalog No.:BCX1236
CAS No.:89560-97-4
- Hedysarimcoumestan B
Catalog No.:BCX1235
CAS No.:899436-04-5
- 2-(3,4-dihydroxyphenyl)-7-(β-D-glucopyranosyloxy)-8-hydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX1234
CAS No.:925701-05-9
- Sikokianin E
Catalog No.:BCX1233
CAS No.:2253791-96-5
- Isocoreopsin
Catalog No.:BCX1232
CAS No.:30382-18-4
- Dihydrobaicalin
Catalog No.:BCX1231
CAS No.:56226-98-3
- Segetalin C
Catalog No.:BCX1230
CAS No.:177602-12-9
- 2,7-dihydroxy-4, 6-dimethoxy phenanthrene
Catalog No.:BCX1229
CAS No.:108352-70-1
- Argentinogenin
Catalog No.:BCX1228
CAS No.:4236-48-0
- β-Obscurin
Catalog No.:BCX1227
CAS No.:467-79-8
- 1-O-gentiobiosyl-3,7-dimethoxy-8-hydroxyxanthone
Catalog No.:BCX1240
CAS No.:487040-33-5
- Chikusaikoside II
Catalog No.:BCX1241
CAS No.:166338-14-3
- Damnacanthol
Catalog No.:BCX1242
CAS No.:477-83-8
- Aconicarchamine B
Catalog No.:BCX1243
CAS No.:1275535-67-5
- Hypolaetin 7-O-glucoside
Catalog No.:BCX1244
CAS No.:32455-43-9
- Hypoletin-7-O-β-D-xylopyranoside
Catalog No.:BCX1245
CAS No.:126771-28-6
- Carmichasine B
Catalog No.:BCX1246
CAS No.:2245700-60-9
- trans-Ferulic acid-4-β-glucoside
Catalog No.:BCX1247
CAS No.:117405-51-3
- 16β-Hydroperoxyalisol B 23-acetate
Catalog No.:BCX1248
CAS No.:2221029-54-3
- 11(α)-Hydroxynepasaikosaponin k
Catalog No.:BCX1249
CAS No.:1152168-63-2
- D-Gluconic acid, 6-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]
Catalog No.:BCX1250
CAS No.:1147861-80-0
- Betulone
Catalog No.:BCX1251
CAS No.:7020-34-0
Anticholinesterase Compounds from Endemic Prangos uechtritzii.[Pubmed:36201258]
Chem Biodivers. 2022 Nov;19(11):e202200557.
In this study, the anticholinesterase effects of the extracts and isolated compounds from the roots of endemic Prangos uechtritzii Boiss & Hausskn (Apiaceae) are reported. A novel polyacetylenic compound; (+)-8-O-methyloplopantriol A along with two known polyacetylenes; (-)-panaxynol, (+)-falcarindiol and fifteen known coumarin derivatives; umbelliferone, 6-formylumbelliferone, suberosin, 7-demethylsuberosin, (+)-ulopterol, tamarin, psoralen, imperatorin, (+)-oxypeucedanin, (+)-oxypeucedanin hydrate, (+)-Oxypeucedanin methanolate, (+)-marmesin, (-)-prantschimgin, (+)-decursinol, and (-)-adicardin were isolated from the hexane (Pu-HE), chloroform (Pu-CE), and methanol (Pu-ME) extracts of P. uechtritzii roots. (-)-Panaxynol, (+)-falcarindiol, 6-formylumbelliferone, (+)-decursinol, and (-)-adicardin were obtained from the genus Prangos for the first time. (+)-8-O-Methyloplopantriol A inhibited both AChE (IC(50) =194.5+/-5.8 muM) and BChE (IC(50) =51.9+/-2.96 muM) enzymes. (+)-Falcarindiol, 6-formylumbelliferone, 7-demethylsuberosin, tamarin, and imperatorin also exhibited BChE-specific inhibitory activities (IC(50) =27.88-93.86 muM). (+)-Falcarindiol (IC(50) =27.88+/-0.91 muM) and imperatorin (IC(50) =30.89+/-1.40 muM) as the most active components could be led compounds to develop new BChE inhibitors with further research against Alzheimer's disease.
Antiproliferative and cytotoxic activities of furocoumarins of Ducrosia anethifolia.[Pubmed:31070540]
Pharm Biol. 2018 Dec;56(1):658-664.
CONTEXT: Phytochemical and pharmacological data on Ducrosia anethifolia (DC.) Boiss. (Apiaceae), an Iranian medicinal plant, are scarce; however, furocoumarins are characteristic compounds of D. anethifolia. OBJECTIVE: Our experiments identify the secondary metabolites of D. anethifolia and assess their antitumor and anti-multidrug resistance activities. MATERIALS AND METHODS: Pure compounds were isolated from the extract of aerial parts of the plant by chromatographic methods. Bioactivities were tested on multidrug resistant and sensitive mouse T-lymphoma cell lines. The inhibition of the cancer MDR efflux pump ABCB1 was evaluated by flow cytometry (at 2 and 20 microM). A checkerboard microplate method was applied to study the interactions of furocoumarins and doxorubicin. Toxicity was studied using normal murine NIH/3T3 fibroblasts. RESULTS: Thirteen pure compounds were isolated, nine furocoumarins namely, pabulenol (1), (+)-oxypeucedanin hydrate (2), oxypeucedanin (3), Oxypeucedanin methanolate (4), (-)-oxypeucedanin hydrate (5), imperatorin (6), isogospherol (7), heraclenin (8), heraclenol (9), along with vanillic aldehyde (10), harmine (11), 3-hydroxy-alpha-ionone (12) and 2-C-methyl-erythrytol (13). Oxypeucedanin showed the highest in vitro antiproliferative and cytotoxic activity against parent (IC(50) = 25.98 +/- 1.27, 40.33 +/- 0.63 microM) and multidrug resistant cells (IC(50) = 28.89 +/- 0.73, 66.68 +/- 0.00 microM), respectively, and exhibited slight toxicity on normal murine fibroblasts (IC(50) = 57.18 +/- 3.91 microM). DISCUSSION AND CONCLUSIONS: Compounds 2, 3, 5, 7, 10-13 were identified for the first time from the Ducrosia genus. Here, we report a comprehensive in vitro assessment of the antitumor activities of D. anethifolia furocoumarins. Oxypeucedanin is a promising compound for further investigations for its anticancer effects.
Bioactive coumarins from the roots and fruits of Ferulago trifida Boiss., an endemic species to Iran.[Pubmed:28954543]
Nat Prod Res. 2018 Nov;32(22):2724-2728.
Phytochemical analysis of the Ferulago trifida Boiss. from Apiaceae family led to the isolation and identification of suberosin (1), isoimperatorin (2), prantschimgin (3), oxypeucedanin (4), Oxypeucedanin methanolate (5), suberenol (6), 6-hydroxymethylherniarin (7), oxypeucedanin hydrate (8), ulopterol (9), bergapten (10), xanthotoxin (11), imperatorin (12) and grandivittin (13) from chloroform extracts of the roots (1-9) and fruits (1, 2, 8, 10-13) of this species. Oxypeucedanin methanolate and suberenol demonstrated a potent antioxidant power with 268.2 +/- 5.4 and 251.2 +/- 6.2 mmol FSE/100 g, respectively, compared by BHT (267.2 +/- 4.2 mmol FSE/100 g) in FRAP method. The potent antibacterial effects were found for Oxypeucedanin methanolate on S. epidermidis (IZ; 26 mm, MIC; 250 mug mL(-1)) an oxypeucedanin hydrate on K. pneumoniae (IZ: 21 mm, MIC: 250 mug mL(-1)). Moreover, suberosin showed higher preferential toxicity against MDA-MB-23 cells (IC(50): 0.21 mM, SI: 5.0), in comparison with tamoxifen (IC(50): 0.012 mM, SI: 2.45) in MTT assay.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica var. formosana cv. Chuanbaizhi].[Pubmed:26552172]
Zhongguo Zhong Yao Za Zhi. 2015 Jun;40(11):2148-56.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica var. formosana cv. Chuanbaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Twenty-nine compounds were obtained and identified as isoimperatorin (1), beta-sitosterol (2), imperatorin (3), bergapten (4), osthenol (5), xanthotoxin (6), isoimpinellin (7), dehydrogeijerin (8), phellopterin (9), isodemethylfuropinarine (10), 7-demethylsuberosin (11), alloimperatorin (12), xanthotoxol (13), isooxypeucedanin (14), alloisoimperatorin (15), demethylfuropinarine (16), 5-hydroxy-8-methoxypsoralen (17), Oxypeucedanin methanolate (18), pabulenol (19), byakangelicin (20), marmesin (21), (+) -decursinol (22), heraclenol (23), oxypeucedanin hydrate (24), marmesinin (25), ulopterol (26), erythro-guaiacylglycerol-beta-ferulic acid ether (27), threo-guaiacylglycerol-beta-ferulic acid ether (28), and uracil (29). Compounds 5, 8, 11, 18, 21-23, and 26-28 were obtained from the roots of title plant for the first time.
Phenylpropanoids and furanocoumarins as antibacterial and antimalarial constituents of the Bhutanese medicinal plant Pleurospermum amabile.[Pubmed:25230503]
Nat Prod Commun. 2014 Jul;9(7):957-60.
With the objective of determining safety and verifying the traditional uses of the Bhutanese medicinal plant, Pleurospermum amabile Craib & W. W. Smith, we investigated its crude extracts and the isolated phytochemicals for their biological activities. Four phenylpropanoids [(E)-isomyristicin (1), (E)-isoapiol (2), methyl eugenol (3) and (E)-isoelemicin (4)] and six furanocoumarins [psoralen (5), bergapten (6), isoimperatorin (7), isopimpinellin (8), oxypeucedanin hydrate (9) and Oxypeucedanin methanolate (10)] were isolated from this plant. Among the test samples, compound 10 showed weak antibacterial activity against Bacillus subtilis and best antimalarial activity against the Plasmodium falciparum strains, TM4/8.2 (chloroquine and antifolate sensitive) and K1CB1 (multidrug resistant). None of the test samples showed cytotoxicity. This study generated scientific data that support the traditional medical uses of the plant.


