IsocoreopsinCAS# 30382-18-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30382-18-4 | SDF | Download SDF |
| PubChem ID | 193124.0 | Appearance | Powder |
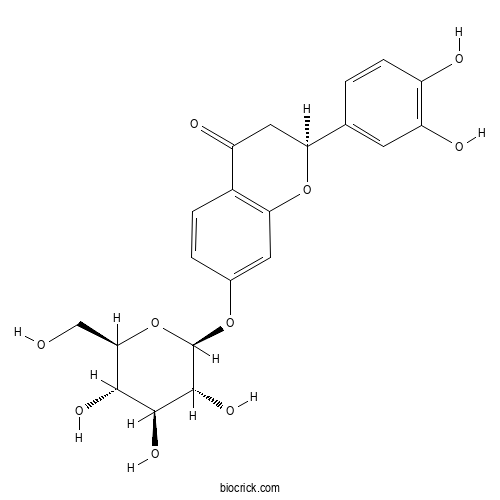
| Formula | C21H22O10 | M.Wt | 434.4 |
| Type of Compound | Dihydroflavones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(3,4-dihydroxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2,3-dihydrochromen-4-one | ||
| SMILES | C1C(OC2=C(C1=O)C=CC(=C2)OC3C(C(C(C(O3)CO)O)O)O)C4=CC(=C(C=C4)O)O | ||
| Standard InChIKey | AWENDZQUFCJISN-ZRWXNEIDSA-N | ||
| Standard InChI | InChI=1S/C21H22O10/c22-8-17-18(26)19(27)20(28)21(31-17)29-10-2-3-11-13(24)7-15(30-16(11)6-10)9-1-4-12(23)14(25)5-9/h1-6,15,17-23,25-28H,7-8H2/t15-,17+,18+,19-,20+,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isocoreopsin Dilution Calculator

Isocoreopsin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.302 mL | 11.5101 mL | 23.0203 mL | 46.0405 mL | 57.5506 mL |
| 5 mM | 0.4604 mL | 2.302 mL | 4.6041 mL | 9.2081 mL | 11.5101 mL |
| 10 mM | 0.2302 mL | 1.151 mL | 2.302 mL | 4.6041 mL | 5.7551 mL |
| 50 mM | 0.046 mL | 0.2302 mL | 0.4604 mL | 0.9208 mL | 1.151 mL |
| 100 mM | 0.023 mL | 0.1151 mL | 0.2302 mL | 0.4604 mL | 0.5755 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydrobaicalin
Catalog No.:BCX1231
CAS No.:56226-98-3
- Segetalin C
Catalog No.:BCX1230
CAS No.:177602-12-9
- 2,7-dihydroxy-4, 6-dimethoxy phenanthrene
Catalog No.:BCX1229
CAS No.:108352-70-1
- Argentinogenin
Catalog No.:BCX1228
CAS No.:4236-48-0
- β-Obscurin
Catalog No.:BCX1227
CAS No.:467-79-8
- Cnidimol B
Catalog No.:BCX1226
CAS No.:103629-81-8
- Erythro-guaiacylglycerol-β-O-4'-sinapyl ether
Catalog No.:BCX1225
CAS No.:905726-70-7
- threo-guaiacylglycerol-β-O-4'-sinapyl ether
Catalog No.:BCX1224
CAS No.:288864-26-6
- 5,7-Dihydroxychroman-4-one
Catalog No.:BCX1223
CAS No.:108085-46-7
- 7,3',5'-Trihydroxyflavanone
Catalog No.:BCX1222
CAS No.:847375-46-6
- 7'R,8'R-2,2'-Dimethoxy-4-(3-hydroxyl-propenyl)-4'-(1,2,3-trihydroxy-propyl) biphenyl ether
Catalog No.:BCX1221
CAS No.:515813-60-2
- 7,8,3',4'-Tetrahydroxyflavanone
Catalog No.:BCX1220
CAS No.:489-73-6
- Sikokianin E
Catalog No.:BCX1233
CAS No.:2253791-96-5
- 2-(3,4-dihydroxyphenyl)-7-(β-D-glucopyranosyloxy)-8-hydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX1234
CAS No.:925701-05-9
- Hedysarimcoumestan B
Catalog No.:BCX1235
CAS No.:899436-04-5
- t-OMe-Byakangelicin
Catalog No.:BCX1236
CAS No.:89560-97-4
- Albanol B
Catalog No.:BCX1237
CAS No.:87084-99-9
- Ekersenin
Catalog No.:BCX1238
CAS No.:53091-74-0
- Oxypeucedanin methanolate
Catalog No.:BCX1239
CAS No.:52939-12-5
- 1-O-gentiobiosyl-3,7-dimethoxy-8-hydroxyxanthone
Catalog No.:BCX1240
CAS No.:487040-33-5
- Chikusaikoside II
Catalog No.:BCX1241
CAS No.:166338-14-3
- Damnacanthol
Catalog No.:BCX1242
CAS No.:477-83-8
- Aconicarchamine B
Catalog No.:BCX1243
CAS No.:1275535-67-5
- Hypolaetin 7-O-glucoside
Catalog No.:BCX1244
CAS No.:32455-43-9
In silico analysis of polyphenols and flavonoids for design of human Nav1.7 inhibitors.[Pubmed:32686994]
J Biomol Struct Dyn. 2021 Aug;39(12):4472-4479.
Neuropathic pain is commonly associated with lesion or disease of the somatosensory system and often reflected as indicator of impaired life. Although the central nervous system is main regulator of pain but for initiation and maintenance of the neuropathic pain is regulated by peripheral nervous system. Sodium channels particularly Nav1.7, Nav1.8, Nav 1.9 are key stake holders in the peripheral neuropathy, activation of these sodium channels might lead to genesis and propagation. Flavonoids and polyphenols showed promising effects in neuropathic pain. Here we are reporting In silico analysis of some selected flavonoids and polyphenols on sodium activated voltage channel 1.7 to explore the structural fragments required for binding. Results indicated Baicalin, Butrin, Dihydromonospermoside, Icariin, Isocoreopsin and Isosaponarin are showing promising docking score with sodium activated voltage channel 1.7 than other compounds. Structural modification of these promising leads keeping pharamcophoric requirement intact may yield potent Nav1.7 inhibitors for peripheral pain management.Communicated by Ramaswamy H. Sarma.
Isocoreopsin: An active constituent of n-butanol extract of Butea monosperma flowers against colorectal cancer (CRC).[Pubmed:29403999]
J Pharm Anal. 2016 Oct;6(5):318-325.
The herb Butea monosperma constitutes several human health beneficial components, which are mostly studied for their anticancer effects. In this study, the activity of n-butanol fractions of B. monosperma floral extract was examined on inhibiting aberrant crypt foci (ACF) formation in azoxymethane induced Wistar albino rats. The n-butanol extracts (150 mg/kg) decreased the ACF formation (per rat) by 92% and 78% in short- and long-term in vivo treatments, respectively. All the compounds in the n-butanol extract were isolated and purified using column and reverse-phase high pressure liquid chromatography (HPLC). Their structures were characterized using UV-visible spectroscopy, nuclear magnetic resonance (NMR) and electrospray-ionisation mass spectrometry (ESI-MS) to determine important flavonoids, namely Isocoreopsin, butrin and isobutrin. These compounds were studied for their free radical scavenging and anticancer activities. The compound Isocoreopsin showed significantly greater efficacy in cell death on human colon and liver cancer cell lines (50 mug/mL in HT-29 and 100 mug/mL in HepG2) than butrin (100 mug/mL in HT-29 and 500 mug/mL in HepG2) and isobutrin (80 mug/mL in HT-29 and 150 mug/mL in HepG2). These results suggest that Isocoreopsin, butrin and isobutrin are the important key compounds for the chemoprevention of colon cancer and Isocoreopsin can be considered as a promising novel drug.
Chemical characteristics of different parts of Coreopsis tinctoria in China using microwave-assisted extraction and high-performance liquid chromatography followed by chemometric analysis.[Pubmed:27291468]
J Sep Sci. 2016 Aug;39(15):2919-27.
Coreopsis tinctoria, also called "snow chrysanthemum" in China, is a flower tea material that has been reported to possess excellent pharmacological properties such as antioxidant and antidiabetic activities. The chemical characteristics of different parts (flowers, buds, seeds, stems, and leaves) of C. tinctoria were investigated based on microwave-assisted extraction and the simultaneous determination of 13 major active compounds by high-performance liquid chromatography, including taxifolin-7-O-glucoside, chlorogenic acid, (R/S)-flavanomarein, Isocoreopsin, quercetagetin-7-O-glucoside, isookanin, 5,7,3',5'-tetrahydroxyflavanone-7-O-glucoside, marein, 3,5-dicaffeoylquinic acid, coreopsin, okanin, 5,7,3',5'-tetrahydroxyflavanone, and N(1) ,N(5) ,N(10) ,N(14) -tetra-p-coumaroylspermine. Chemometric analysis based on the contents of investigated compounds from 13 samples showed that C. tinctoria and the related flower tea materials, Chrysanthemum morifolium cv "Hangju" and "Gongju," were in different clusters, and different parts (flowers, buds, seeds, stems, and leaves) of C. tinctoria were obviously different. This study is helpful for the quality control and pharmacological evaluation of different parts from C. tinctoria and its related products.
Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-kappaB in activated human mast cells: suppression of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8.[Pubmed:20164300]
J Pharmacol Exp Ther. 2010 May;333(2):354-63.
Activation of mast cells in rheumatoid synovial tissue has often been associated with tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, and IL-8 production and disease pathogenesis by adjacent cell types. Butea monosperma (BM) is a well known medicinal plant in India and the tropics. The aim of this study was to examine whether a standardized extract of BM flower (BME) could inhibit inflammatory reactions in human mast cells (HMC) using activated HMC-1 cells as a model. Four previously characterized polyphenols--butrin, isobutrin, Isocoreopsin, and butein--were isolated from BME by preparative thin layer chromatography, and their purity and molecular weights were determined by liquid chromatography/mass spectrometry analysis. Our results showed that butrin, isobutrin, and butein significantly reduced the phorbol 12-myristate 13-acetate and calcium ionophore A23187-induced inflammatory gene expression and production of TNF-alpha, IL-6, and IL-8 in HMC-1 cells by inhibiting the activation of NF-kappaB. In addition, isobutrin was most potent in suppressing the NF-kappaB p65 activation by inhibiting IkappaBalpha degradation, whereas butrin and butein were relatively less effective. In vitro kinase activity assay revealed that isobutrin was a potent inhibitor of IkappaB kinase complex activity. This is the first report identifying the molecular basis of the reported anti-inflammatory effects of BME and its constituents butrin, isobutrin, and butein. The novel pharmacological actions of these polyphenolic compounds indicate potential therapeutic value for the treatment of inflammatory and other diseases in which activated mast cells play a role.


