AlternariolCAS# 641-38-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 641-38-3 | SDF | Download SDF |
| PubChem ID | 5359485 | Appearance | Powder |
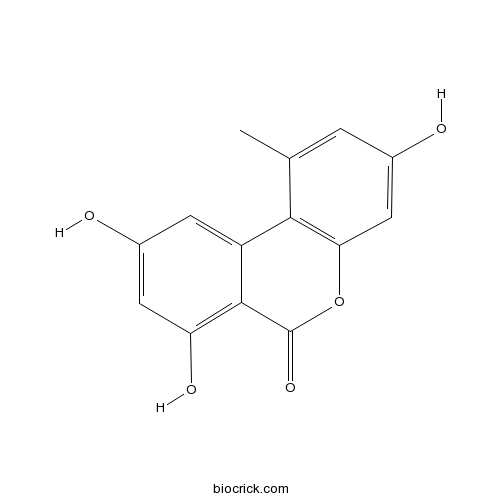
| Formula | C14H10O5 | M.Wt | 258.22 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,7,9-trihydroxy-1-methylbenzo[c]chromen-6-one | ||
| SMILES | CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)O)O | ||
| Standard InChIKey | CEBXXEKPIIDJHL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10O5/c1-6-2-7(15)5-11-12(6)9-3-8(16)4-10(17)13(9)14(18)19-11/h2-5,15-17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Alternariol, a mycotoxin, is found in food and beverages infected by Alternaria alternata, it shows estrogenic potential, inhibition of cell proliferation and clastogenicity in Ishikawa and V79 cells in vitro. 2. Alternariol possesses genotoxic properties, it is a poison of topoisomerase I and II with a certain selectivity for the II isoform. 3. Alternariol has strong activities against KB and KBv200 cells with IC 50 values of 3.17, 3.12 ug/mL. 4. Alternariol induces abnormal nuclear morphology and cell cycle arrest in murine RAW 264.7 macrophages. 5. Alternariol is a new phototoxic, DNA-intercalating agent and is a DNA cross-linking mycotoxin in near UV light. |
| Targets | Topoisomerase |

Alternariol Dilution Calculator

Alternariol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8727 mL | 19.3633 mL | 38.7267 mL | 77.4533 mL | 96.8167 mL |
| 5 mM | 0.7745 mL | 3.8727 mL | 7.7453 mL | 15.4907 mL | 19.3633 mL |
| 10 mM | 0.3873 mL | 1.9363 mL | 3.8727 mL | 7.7453 mL | 9.6817 mL |
| 50 mM | 0.0775 mL | 0.3873 mL | 0.7745 mL | 1.5491 mL | 1.9363 mL |
| 100 mM | 0.0387 mL | 0.1936 mL | 0.3873 mL | 0.7745 mL | 0.9682 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sennidin A
Catalog No.:BCN6354
CAS No.:641-12-3
- H-Ala(3-pyridyl)-OH.HCl
Catalog No.:BCC3321
CAS No.:64090-98-8
- Cannabispirol
Catalog No.:BCN4609
CAS No.:64052-90-0
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- Ro 04-5595 hydrochloride
Catalog No.:BCC7234
CAS No.:64047-73-0
- Z-D-Pro-OH
Catalog No.:BCC2752
CAS No.:6404-31-5
- Boc-ε-Acp-OH
Catalog No.:BCC3205
CAS No.:6404-29-1
- Boc-Nle-OH
Catalog No.:BCC3296
CAS No.:6404-28-0
- Boc-Lys(Ac)-OH
Catalog No.:BCC3411
CAS No.:6404-26-8
- Torachrysone 8-O-glucoside
Catalog No.:BCN4181
CAS No.:64032-49-1
- 4(15)-Oppositene-1,7-diol
Catalog No.:BCN4180
CAS No.:640289-58-3
- Tamgermanetin
Catalog No.:BCN7944
CAS No.:640235-90-1
- Allomatrine
Catalog No.:BCN2368
CAS No.:641-39-4
- 5-hydroxy-canthin-6-one
Catalog No.:BCN7910
CAS No.:64118-73-6
- Di-O-methylcrenatin
Catalog No.:BCN4608
CAS No.:64121-98-8
- Longistylin C
Catalog No.:BCN4182
CAS No.:64125-60-6
- Sedanolide
Catalog No.:BCN8338
CAS No.:6415-59-4
- Nilotinib(AMN-107)
Catalog No.:BCC3643
CAS No.:641571-10-0
- Echinoynethiophene A
Catalog No.:BCN4183
CAS No.:64165-98-6
- 5-Acetyl-2-(1-hydroxy-1-methylethyl)benzofuran
Catalog No.:BCN7488
CAS No.:64165-99-7
- 30-Oxolupeol
Catalog No.:BCN6673
CAS No.:64181-07-3
- trans-2,3-Dihydro-3-hydroxyeuparin
Catalog No.:BCN6922
CAS No.:64185-57-5
- Z-Hyp-Ome
Catalog No.:BCC3258
CAS No.:64187-48-0
- Zanthobungeanine
Catalog No.:BCN6685
CAS No.:64190-94-9
Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells.[Pubmed:16194592]
Food Chem Toxicol. 2006 Mar;44(3):398-408.
The mycotoxin Alternariol (AOH) is found in food and beverages infected by Alternaria alternata. Because consumption of foodstuffs contaminated with A. alternata has been implicated in an elevated incidence of esophageal carcinogenesis, we have investigated the estrogenic potential, the effect on cell proliferation, and the genotoxic effect of AOH in cultured mammalian cells. AOH replaced E2 from isolated human estrogen receptors alpha and beta and increased the level of alkaline phosphatase (ALP) mRNA and the enzymatic activity of ALP in a human endometrial adenocarcinoma cell line (Ishikawa cells). The estrogenicity of AOH was about 0.01% of that of E2. The effects in Ishikawa cells were reversed by the ER antagonist ICI 182,780. Analysis of cell proliferation by flow cytometry and microscopy of Ishikawa and Chinese hamster V79 cells revealed that AOH inhibited cell proliferation by interference with the cell cycle. The genotoxic potential was assessed by the micronucleus (MN) assay and immunochemical differentiation between MN containing whole chromosomes (kinetochore-positive) and DNA fragments (kinetochore-negative) in Ishikawa and V79 cells. AOH induced kinetochore-negative MN in both cell lines. This is the first report on the estrogenic potential, inhibition of cell proliferation and clastogenicity of AOH in Ishikawa and V79 cells in vitro.
Alternariol acts as a topoisomerase poison, preferentially affecting the IIalpha isoform.[Pubmed:18727009]
Mol Nutr Food Res. 2009 Apr;53(4):441-51.
Alternariol (AOH), a mycotoxin formed by Alternaria alternata, has been reported to possess genotoxic properties. However, the underlying mechanism of action is unclear. Here, we tested the hypothesis that interactions with DNA-topoisomerases play a role in the DNA-damaging properties of AOH. First we compared DNA-damaging properties of AOH with other Alternaria mycotoxins such as AOH monomethyl ether (AME), altenuene and isoaltenuene. AOH and AME significantly increased the rate of DNA strand breaks in human carcinoma cells (HT29, A431) at micromolar concentrations, whereas altenuene and isoaltenuene did not affect DNA integrity up to 100 microM. Next, we selected AOH as the most DNA-damaging Alternaria metabolite for further studies of interactions with DNA topoisomerases. In cell-free assays, AOH potently inhibited DNA relaxation and stimulated DNA cleavage activities of topoisomerase I, IIalpha and IIbeta. Stabilisation of covalent topoisomerase II-DNA intermediates by AOH was also detectable in cell culture, and here, the IIalpha isoform was preferentially targeted. AOH is thus characterised as a poison of topoisomerase I and II with a certain selectivity for the IIalpha isoform. Since topoisomerase poisoning and DNA strand breakage occurred within the same concentration range, poisoning of topoisomerase I and II might at least contribute to the genotoxic properties of AOH.
Alternariol, a dibenzopyrone mycotoxin of Alternaria spp., is a new photosensitizing and DNA cross-linking agent.[Pubmed:3899714]
Experientia. 1985 Sep 15;41(9):1188-90.
The mycotoxin Alternariol (3,4',5-trihydroxy-6'-methyldibenzo [a] pyrone) but not Alternariol monomethyl ether (3,4'-dihydroxy-5-methoxy-6'-methyldibenzo [a] pyrone) is phototoxic to Escherichia coli in the presence of near UV light (320-400 nm). The phototoxicity bioassays with a DNA repair-deficient mutant of E. coli suggested that DNA may be the molecular target for photo-induced toxicity of Alternariol. Interactions between Alternariol and double-stranded, supercoiled DNA suggest that Alternariol interacts with DNA by intercalation. No DNA breakage was detected in this system; however, Alternariol forms a complex and cross-links double-stranded DNA in near UV light. These results suggest that Alternariol is a new phototoxic, DNA-intercalating agent and is a DNA cross-linking mycotoxin in near UV light.
Alternariol induces abnormal nuclear morphology and cell cycle arrest in murine RAW 264.7 macrophages.[Pubmed:23454835]
Toxicol Lett. 2013 May 10;219(1):8-17.
The mycotoxin Alternariol (AOH), a frequent contaminant in fruit and cereal products, is known to induce DNA damage with subsequent cell cycle arrest. Here we elucidated the effects of AOH on stages of cell cycle progression using the RAW 264.7 macrophage model. AOH resulted in an accumulation of cells in the G2/M-phase (4N). Most cells exhibited a large G2 nucleus whereas numbers of true mitotic cells were reduced relative to control. Both cyclin B1 and p-cdc2 levels increased, while cyclin B1 remained in the cytoplasm; suggesting arrest in the G2/M transition point. Remarkably, after exposure to AOH for 24h, most of the cells exhibited abnormally shaped nuclei, as evidenced by partly divided nuclei, nuclear blebs, polyploidy and micronuclei (MN). AOH treatment also induced abnormal Aurora B bridges, suggesting that cytokinesis was interfered within cells undergoing karyokinesis. A minor part of the resultant G1 tetraploid (4N) cells re-entered the S-phase and progressed to 8N cells.


