CannabispirolCAS# 64052-90-0 |
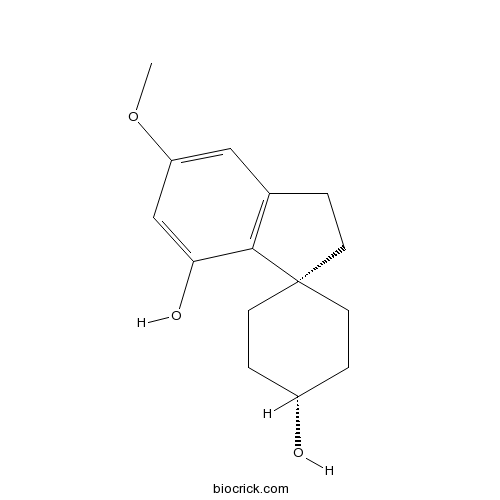
- α-Cannabispiranol
Catalog No.:BCX1628
CAS No.:69636-83-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 64052-90-0 | SDF | Download SDF |
| PubChem ID | 194174 | Appearance | Powder |
| Formula | C15H20O3 | M.Wt | 248.32 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | beta-Cannabispiranol;69636-83-5;alpha-Cannabispiranol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-methoxyspiro[1,2-dihydroindene-3,4'-cyclohexane]-1',4-diol | ||
| SMILES | COC1=CC(=C2C(=C1)CCC23CCC(CC3)O)O | ||
| Standard InChIKey | ZFFYHYZOCYEEPL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H20O3/c1-18-12-8-10-2-5-15(14(10)13(17)9-12)6-3-11(16)4-7-15/h8-9,11,16-17H,2-7H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cannabispirol has a weak antibacterial effect which is more definite on plasmid carrying bacteria than plasmidless ones, and inhibits intercellular plasmid transfer and transforming activity of plasmid DNA. |
| Targets | Antifection |

Cannabispirol Dilution Calculator

Cannabispirol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0271 mL | 20.1353 mL | 40.2706 mL | 80.5412 mL | 100.6765 mL |
| 5 mM | 0.8054 mL | 4.0271 mL | 8.0541 mL | 16.1082 mL | 20.1353 mL |
| 10 mM | 0.4027 mL | 2.0135 mL | 4.0271 mL | 8.0541 mL | 10.0677 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8054 mL | 1.6108 mL | 2.0135 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8054 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- Ro 04-5595 hydrochloride
Catalog No.:BCC7234
CAS No.:64047-73-0
- Z-D-Pro-OH
Catalog No.:BCC2752
CAS No.:6404-31-5
- Boc-ε-Acp-OH
Catalog No.:BCC3205
CAS No.:6404-29-1
- Boc-Nle-OH
Catalog No.:BCC3296
CAS No.:6404-28-0
- Boc-Lys(Ac)-OH
Catalog No.:BCC3411
CAS No.:6404-26-8
- Torachrysone 8-O-glucoside
Catalog No.:BCN4181
CAS No.:64032-49-1
- 4(15)-Oppositene-1,7-diol
Catalog No.:BCN4180
CAS No.:640289-58-3
- Tamgermanetin
Catalog No.:BCN7944
CAS No.:640235-90-1
- Paroxetine maleate
Catalog No.:BCC7265
CAS No.:64006-44-6
- H-D-Val-OH
Catalog No.:BCC3145
CAS No.:640-68-6
- Etimizol
Catalog No.:BCC1562
CAS No.:64-99-3
- H-Ala(3-pyridyl)-OH.HCl
Catalog No.:BCC3321
CAS No.:64090-98-8
- Sennidin A
Catalog No.:BCN6354
CAS No.:641-12-3
- Alternariol
Catalog No.:BCN7147
CAS No.:641-38-3
- Allomatrine
Catalog No.:BCN2368
CAS No.:641-39-4
- 5-hydroxy-canthin-6-one
Catalog No.:BCN7910
CAS No.:64118-73-6
- Di-O-methylcrenatin
Catalog No.:BCN4608
CAS No.:64121-98-8
- Longistylin C
Catalog No.:BCN4182
CAS No.:64125-60-6
- Sedanolide
Catalog No.:BCN8338
CAS No.:6415-59-4
- Nilotinib(AMN-107)
Catalog No.:BCC3643
CAS No.:641571-10-0
- Echinoynethiophene A
Catalog No.:BCN4183
CAS No.:64165-98-6
- 5-Acetyl-2-(1-hydroxy-1-methylethyl)benzofuran
Catalog No.:BCN7488
CAS No.:64165-99-7
- 30-Oxolupeol
Catalog No.:BCN6673
CAS No.:64181-07-3
Non-cannabinoid constituents from a high potency Cannabis sativa variety.[Pubmed:18774146]
Phytochemistry. 2008 Oct;69(14):2627-33.
Six new non-cannabinoid constituents were isolated from a high potency Cannabis sativa L. variety, namely 5-acetoxy-6-geranyl-3-n-pentyl-1,4-benzoquinone (1), 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene (2), 4-hydroxy-2,3,6,7-tetramethoxy-9,10-dihydrophenanthrene (3), 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene (4), cannflavin C (5) and beta-sitosteryl-3-O-beta-d-glucopyranoside-2'-O-palmitate (6). In addition, five known compounds, alpha-cannabispiranol (7), chrysoeriol (8), 6-prenylapigenin (9), cannflavin A (10) and beta-acetyl cannabispiranol (11) were identified, with 8 and 9 being reported for the first time from cannabis. Some isolates displayed weak to strong antimicrobial, antileishmanial, antimalarial and anti-oxidant activities. Compounds 2-4 were inactive as analgesics.
The effects of cannabispiro compounds and tetrahydrocannabidiolic acid on the plasmid transfer and maintenance in Escherichia coli.[Pubmed:3551476]
Acta Microbiol Hung. 1986;33(3):221-31.
Some cannabispiro compounds and tetrahydrocannabidiolic acid were tested for antibacterial plasmid curing activity and inhibition of plasmid transfer. MIC values of the compound were above 1500 micrograms/ml. Cannabispirol and tetrahydrocannabidiolic acid eliminated the F'lac plasmid from Escherichia coli, but acetylCannabispirol, cannabispirone and cannabispirenone were ineffective as curing agents. Each compound, except acetyl-Cannabispirol, selectively killed plasmid carrying bacteria. The compounds inhibited R144 plasmid transfer from E. coli into E. coli cells via inhibition of mating pair formation, zygotic killing and inhibition of transconjugal DNA synthesis in a lesser extent. All of the cannabispiro compounds and tetrahydrocannabidiolic acid inhibited the transformation with pBR322 plasmid DNA when the bacteria were pretreated with the compounds, via inhibition of the DNA penetration or decreasing the synthesis of plasmid DNA during bacterial growth. Although each of the compounds, except acetyl-Cannabispirol, had a weak antibacterial effect which was more definite on plasmid carrying bacteria than plasmidless ones, and inhibited intercellular plasmid transfer and transforming activity of plasmid DNA, only two of them were able to cure F'lac plasmid showing that plasmid elimination is a complex process which strictly depends on the stereochemical configuration of curing agents.


