BiflorinCAS# 89701-85-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 89701-85-9 | SDF | Download SDF |
| PubChem ID | 441959 | Appearance | Powder |
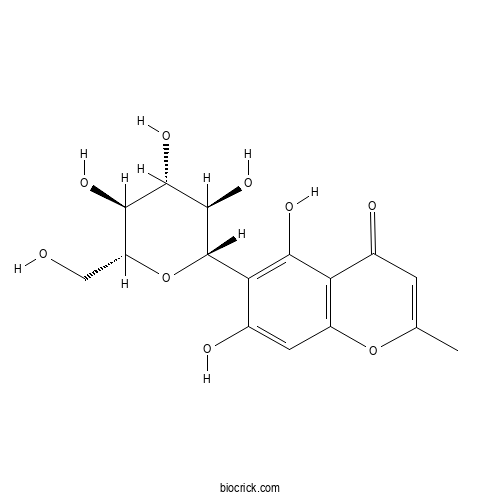
| Formula | C16H18O9 | M.Wt | 354.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,7-dihydroxy-2-methyl-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one | ||
| SMILES | CC1=CC(=O)C2=C(O1)C=C(C(=C2O)C3C(C(C(C(O3)CO)O)O)O)O | ||
| Standard InChIKey | XTZWWMZDVUKEDJ-SPEJKDPOSA-N | ||
| Standard InChI | InChI=1S/C16H18O9/c1-5-2-6(18)10-8(24-5)3-7(19)11(13(10)21)16-15(23)14(22)12(20)9(4-17)25-16/h2-3,9,12,14-17,19-23H,4H2,1H3/t9-,12-,14+,15-,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Biflorin Dilution Calculator

Biflorin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8225 mL | 14.1123 mL | 28.2247 mL | 56.4493 mL | 70.5617 mL |
| 5 mM | 0.5645 mL | 2.8225 mL | 5.6449 mL | 11.2899 mL | 14.1123 mL |
| 10 mM | 0.2822 mL | 1.4112 mL | 2.8225 mL | 5.6449 mL | 7.0562 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5645 mL | 1.129 mL | 1.4112 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5645 mL | 0.7056 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3alpha-Hinokiol
Catalog No.:BCX2114
CAS No.:107740-34-1
- Tellimagrandin I
Catalog No.:BCX2113
CAS No.:79786-08-6
- O-Methylglycosolone
Catalog No.:BCX2112
CAS No.:41303-25-7
- Garcinexanthone C
Catalog No.:BCX2111
CAS No.:1107620-69-8
- Mangostenone F
Catalog No.:BCX2110
CAS No.:1242438-70-5
- cis-N-Terrestriamide
Catalog No.:BCX2109
CAS No.:2170469-93-7
- Morindaparvin W
Catalog No.:BCX2108
CAS No.:2596365-67-0
- 25(27)-Ene-elephanoside H
Catalog No.:BCX2107
CAS No.:1592406-40-0
- Flindersine
Catalog No.:BCX2106
CAS No.:523-64-8
- Isorhamnetin 3-O-apiosyl (1->2)[rhamnosyl (1->6)]glucoside
Catalog No.:BCX2105
CAS No.:165605-18-5
- 25R-Tupistroside E
Catalog No.:BCX2104
CAS No.:1448639-95-9
- Stellarin 2
Catalog No.:BCX2103
CAS No.:63975-58-6
- 6-Hydroxy-2-methoxyacetophenone 4-O-beta-D-xylopyranosyl-(1->6)-beta-D-glucopyranoside
Catalog No.:BCX2116
CAS No.:2140317-59-3
- Fischeroside B
Catalog No.:BCX2117
CAS No.:1307257-08-4
- Isolophanthin B
Catalog No.:BCX2118
CAS No.:1370511-57-1
- 7-O-Methylhorminone
Catalog No.:BCX2119
CAS No.:122482-10-4
- Isocarthamidin 7-O-glucuronide
Catalog No.:BCX2120
CAS No.:119600-61-2
- 6,8-Di-C-beta-D-glucopyranosylacacetin
Catalog No.:BCX2121
CAS No.:28234-99-3
- 1,5-Epoxy-3S-hydroxy-1-(3,4-dihydroxy-5-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane
Catalog No.:BCX2122
CAS No.:182369-54-6
- Callicapoic acid M1
Catalog No.:BCX2123
CAS No.:2756970-65-5
- 8-C-beta-D-(6-O-galloyl)glucosylnoreugenin
Catalog No.:BCX2124
CAS No.:152041-17-3
- 3'-Methoxynyasin
Catalog No.:BCX2125
CAS No.:1685246-20-1
- Isorhamnetin 3-sambubioside
Catalog No.:BCX2126
CAS No.:142059-76-5
- Ellagic acid glucoside
Catalog No.:BCX2127
CAS No.:163774-64-9
Naphthoquinones biflorin and bis-biflorin (Capraria biflora) as possible inhibitors of the fungus Candida auris polymerase: molecular docking, molecular dynamics, MM/GBSA calculations and in silico drug-likeness study.[Pubmed:36597918]
J Biomol Struct Dyn. 2023;41(21):11564-11577.
A new worldwide concern has emerged with the recent emergence of infections caused by Candida auris. This reflects its comparative ease of transmission, substantial mortality, and the increasing level of resistance seen in the three major classes of antifungal drugs. Efforts to create a better design for structure-based drugs that described numerous modifications and the search for secondary metabolic structures derived from plant species are likely to reduce the virulence of several fungal pathogens. In this context, the present work aimed to evaluate in silico two naphthoquinones isolated from the roots of Capraria biflora, Biflorin, and its dimmer, bis-Biflorin, as potential inhibitors of Candida auris polymerase. Based on the simulation performed with the two naphthoquinones, Biflorin and bis-Biflorin, it can be stated that bis-Biflorin showed the best interactions with Candida auris polymerase. Still, Biflorin also demonstrated favorable coupling energy. Predictive pharmacokinetic assays suggest that Biflorin has high oral bioavailability and more excellent metabolic stability compared to the bis-Biflorin analogue. constituting a promising pharmacological tool.Communicated by Ramaswamy H. Sarma.
A Review of Malaysian Herbal Plants and Their Active Constituents with Potential Therapeutic Applications in Sepsis.[Pubmed:33193799]
Evid Based Complement Alternat Med. 2020 Oct 28;2020:8257817.
Sepsis refers to organ failure due to uncontrolled body immune responses towards infection. The systemic inflammatory response triggered by pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) from Gram-negative bacteria, is accompanied by the release of various proinflammatory mediators that can lead to organ damage. The progression to septic shock is even more life-threatening due to hypotension. Thus, sepsis is a leading cause of death and morbidity globally. However, current therapies are mainly symptomatic treatment and rely on the use of antibiotics. The lack of a specific treatment demands exploration of new drugs. Malaysian herbal plants have a long history of usage for medicinal purposes. A total of 64 Malaysian plants commonly used in the herbal industry have been published in Malaysian Herbal Monograph 2015 and Globinmed website (http://www.globinmed.com/). An extensive bibliographic search in databases such as PubMed, ScienceDirect, and Scopus revealed that seven of these plants have antisepsis properties, as evidenced by the therapeutic effect of their extracts or isolated compounds against sepsis-associated inflammatory responses or conditions in in vitro or/and in vivo studies. These include Andrographis paniculata, Zingiber officinale, Curcuma longa, Piper nigrum, Syzygium aromaticum, Momordica charantia, and Centella asiatica. Among these, Z. officinale is the most widely studied plant and seems to have the highest potential for future therapeutic applications in sepsis. Although both extracts as well as active constituents from these herbal plants have demonstrated potential antisepsis activity, the activity might be primarily contributed by the active constituent(s) from each of these plants, which are andrographolide (A. paniculata), 6-gingerol and zingerone (Z. officinale), curcumin (C. longa), piperine and pellitorine (P. nigrum), Biflorin (S. aromaticum), and asiaticoside, asiatic acid, and madecassoside (C. asiatica). These active constituents have shown great antisepsis effects, and further investigations into their clinical therapeutic potential may be worthwhile.
Biflorin inhibits the proliferation of gastric cancer cells by decreasing MYC expression.[Pubmed:31751609]
Toxicol In Vitro. 2020 Mar;63:104735.
Gastric cancer is the third leading cause of cancer-related death worldwide. To evaluate the anticancer potential and molecular mechanism of Biflorin, a prenyl-ortho-naphthoquinone obtained from Capraria biflora L. roots, we used ACP02, a gastric cancer cell line established from a primary diffuse gastric adenocarcinoma. In this study, Biflorin was shown to be a potent cytotoxic agent against ACP02 by Alamar Blue and Trypan Blue assays. Morphological analysis indicated cell death with features of necrosis. Furthermore, a decrease in colony formation, migration and invasion of ACP02 cells was observed after treatment with Biflorin (1.0, 2.5 and 5.0 muM). Regarding the underlying molecular mechanism of Biflorin in ACP02 cells, we observed a decrease in MYC expression and telomere length using FISH. Our findings suggest a novel molecular target of Biflorin in ACP02 cells, which may be a significant therapeutic approach for gastric cancer management.
Screening free radical scavengers in Xiexin Tang by HPLC-ABTS-DAD-Q-TOF/MS.[Pubmed:28477383]
Biomed Chromatogr. 2017 Nov;31(11).
Xiexin Tang (XXT) is a traditional Chinese medicine (TCM) that has been used in herbal clinics for more than 1800 years. Many studies have shown that XXT has therapeutic effects on patients with arteriosclerosis owing to its antioxidant activity. However, there is little information about the relationship between the chemical composition of XXT and its antioxidant activity. In this study, the HPLC-ABTS-DAD-Q-TOF/MS method, which can simultaneously identify individual components and rapidly screen for antioxidant compounds, was used to screen and identify antioxidant components in XXT. The 15 compounds identified were gluco-syringic acid, adenine, gallic acid, Biflorin, cularine, 6-C-arabinose-8-C-glucose-chrysin, 6-C-glucose-8-C-arabinose-chrysin, baicalin, rhein-8-O-beta-d-glucopyranoside, coptisine, epiberberine, jatrorrhizine, norwogonin, 5,7,2'-trihydroxy-6- methoxyflavone and baicalein. In addition, the data showed that the antioxidant activity of peaks 4, 6, and 11 was lower in XXT than in its constituent herbs, while the activity of peaks 1, 2, 3, 5, 7, 8, 10, 12, 13, 14 and 15 was higher in XXT. Compound 5 had the strongest antioxidant activity in XXT, while compound 1 showed the strongest antioxidant activity among its constituent herb. The differences between antioxidant activities of major components of XXT and those of its constituent herbs might be due to the interaction of crude drugs that changes the solubility of active components during the decoction process. The results show that the HPLC-ABTS-DAD-Q-TOF/MS method can successfully combine on-line mass spectrometry with activity detection system. It is a useful tool for the rapid detection and identification of antioxidants, and for quantitative analysis of individual antioxidants in complex mixtures such as plant extracts. Furthermore, this method does not require extensive extract purification and fraction collection.
Biflorin Ameliorates Memory Impairments Induced by Cholinergic Blockade in Mice.[Pubmed:27829270]
Biomol Ther (Seoul). 2017 May 1;25(3):249-258.
To examine the effect of Biflorin, a component of Syzygium aromaticum, on memory deficit, we introduced a scopolamine-induced cognitive deficit mouse model. A single administration of Biflorin increased latency time in the passive avoidance task, ameliorated alternation behavior in the Y-maze, and increased exploration time in the Morris water maze task, indicating the improvement of cognitive behaviors against cholinergic dysfunction. The Biflorin-induced reverse of latency in the scopolamine-treated group was attenuated by MK-801, an NMDA receptor antagonist. Biflorin also enhanced cognitive function in a naive mouse model. To understand the mechanism of Biflorin for memory amelioration, we performed Western blot. Biflorin increased the activation of protein kinase C-zeta and its downstream signaling molecules in the hippocampus. These results suggest that Biflorin ameliorates drug-induced memory impairment by modulation of protein kinase C-zeta signaling in mice, implying that Biflorin could function as a possible therapeutic agent for the treatment of cognitive problems.
Biflorin induces cytotoxicity by DNA interaction in genetically different human melanoma cell lines.[Pubmed:27079618]
Toxicol In Vitro. 2016 Aug;34:237-245.
Cancer is a public health problem and the second leading cause of death worldwide. The incidence of cutaneous melanoma has been notably increasing, resulting in high aggressiveness and poor survival rates. Taking into account the antitumor activity of Biflorin, a substance isolated from Capraria biflora L. roots that is cytotoxic in vitro and in vivo, this study aimed to demonstrate the action of Biflorin against three established human melanoma cell lines that recapitulate the molecular landscape of the disease in terms of genetic alterations and mutations, such as the TP53, NRAS and BRAF genes. The results presented here indicate that Biflorin reduces the viability of melanoma cell lines by DNA interactions. Biflorin causes single and double DNA strand breaks, consequently inhibiting cell cycle progression, replication and DNA repair and promoting apoptosis. Our data suggest that Biflorin could be considered as a future therapeutic option for managing melanoma.
Biflorin, Isolated from the Flower Buds of Syzygium aromaticum L., Suppresses LPS-Induced Inflammatory Mediators via STAT1 Inactivation in Macrophages and Protects Mice from Endotoxin Shock.[Pubmed:26977531]
J Nat Prod. 2016 Apr 22;79(4):711-20.
Two chromone C-glucosides, Biflorin (1) and isoBiflorin (2), were isolated from the flower buds of Syzygium aromaticum L. (Myrtaceae). Here, inhibitory effects of 1 and 2 on lipopolysaccharide (LPS)-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2) in RAW 264.7 macrophages were evaluated, and 1 (IC50 = 51.7 and 37.1 muM, respectively) was more potent than 2 (IC50 > 60 and 46.0 muM). The suppression of NO and PGE2 production by 1 correlated with inhibition of iNOS and COX-2 protein expression. Compound 1 reduced inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) mRNA expression via inhibition of their promoter activities. Compound 1 inhibited the LPS-induced production and mRNA expression of tumor necrosis factor-alpha (TNF-alpha) and interleukin (IL)-6. Furthermore, 1 reduced p-STAT1 and p-p38 expression but did not affect the activity of nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) or activator protein 1 (AP-1). In a mouse model of LPS-induced endotoxemia, 1 reduced the mRNA levels of iNOS, COX-2, and TNF-alpha, and the phosphorylation-mediated activation of the signal transducer and activator of transcription 1 (STAT1), consequently improving the survival rates of mice. Compound 1 showed a significant anti-inflammatory effect on carrageenan-induced paw edema and croton-oil-induced ear edema in rats. The collective data indicate that the suppression of pro-inflammatory gene expression via p38 mitogen-activated protein kinase and STAT1 inactivation may be a mechanism for the anti-inflammatory activity of 1.
O-naphthoquinone isolated from Capraria biflora L. induces selective cytotoxicity in tumor cell lines.[Pubmed:26782390]
Genet Mol Res. 2015 Dec 21;14(4):17472-81.
Biflorin is an o-naphthoquinone isolated from the roots of the plant Capraria biflora L. (Scrophulariaceae). In this study, the cytotoxic effects of Biflorin were verified, and late apoptosis was detected in various cancer cell lines by in situ analysis. The cytotoxicity was further evaluated exclusively for 48 h of treatment in different tumor and non-tumor cell lines (Hep-2, HeLa, HT-29, A-375, and A-549, and HEK-293, respectively). The results indicated that Biflorin induced selective cytotoxicity in tumor cells. HeLa cells were more susceptible to Biflorin, followed by HT-29, A-549, A-375, and Hep-2 at all concentrations (range 5-50 mug/mL), and the highest half-maximal inhibitory concentration IC50 (56.01 +/- 1.17 mug/mL) was observed in HEK-293 cells. Late apoptotic/necrotic events, observed by in situ immunostaining with Annexin V, varied with each cell line; an increase in late apoptotic events was observed corresponding to the increase in Biflorin dosage. Hep-2 cells showed a greater percentage of late apoptotic events among the tumor cell lines when treated with higher concentrations of Biflorin (69.63 +/- 2.28%). The non-tumor HEK-293 line showed greater resistance to late apoptotic events, as well as a lower level of cytotoxicity (77.69 +/- 6.68%) than the tested tumor lines. The data presented indicate that Biflorin showed an important, possibly selective, cytotoxicity against tumor cell lines, thereby revealing a promising novel substance with potential anticancer activity for tumor therapy.
Synthesis, antibacterial and cytotoxic activities of new biflorin-based hydrazones and oximes.[Pubmed:26684850]
Bioorg Med Chem Lett. 2016 Jan 15;26(2):435-439.
Biflorin 1 is a biologically active quinone, isolated from Capraria biflora. Five new Biflorin-based nitrogen derivatives were synthesized, of which two were mixtures of (E)- and (Z)- isomers: (Z)-2a, (Z)-2b, (Z)-3a, (Z)- and (E)-3b, (Z)- and (E)-3c. The antibacterial activity was investigated using the microdilution method for determining the minimum inhibitory concentration (MIC) against six bacterial strains. Tests have shown that these derivatives have potential against all bacterial strains. The cytotoxic activity was also evaluated against three strains of cancer cells, but none of the derivatives showed activity.
A sensitive LC-MS/MS method for the quantitative determination of biflorin in rat plasma and its application to pharmacokinetic studies.[Pubmed:26263054]
J Pharm Biomed Anal. 2015 Nov 10;115:272-6.
A rapid, sensitive and selective liquid chromatography-tandem mass spectrometric method (LC-MS/MS) was developed for the quantification of Biflorin in rat plasma. Using naringin as an internal standard, plasma samples were subjected to a direct protein precipitation process using methanol. Chromatographic separation was achieved on a Gemini C18 column with an isocratic mobile phase consisting of 0.1% formic acid and methanol (50:50, v/v) at a flow rate of 0.5mL/min. Biflorin was analyzed in the multiple reaction monitoring mode with negative electrospray ionization. The precursor/product ion pairs were m/z 353.0/205.0 and m/z 579.0/271.0 for Biflorin and the IS, respectively. The calibration curve was linear over the concentration range of 5-2000ng/mL. The intra- and inter-day precision was less than 7.3% and the accuracy ranged from 96.5 to 103.3%. No significant variation was observed in the stability tests. This method was successfully applied to a pharmacokinetic study of Biflorin after the intravenous and oral administration of Biflorin to rats. The half-life and oral bioavailability of Biflorin were determined as 3.4h and 43%, respectively. This is the first report on the quantitative determination of Biflorin in rat plasma as well as the pharmacokinetic characterization of Biflorin, which should provide a meaningful foundation for further preclinical and clinical applications of Biflorin.
Biological activity and photostability of biflorin micellar nanostructures.[Pubmed:25985360]
Molecules. 2015 May 13;20(5):8595-604.
Capraria biflora L. is a shrub from the Scrophulariaceae family which produces in its roots a compound named Biflorin, an o-naphthoquinone that shows activity against Gram-positive bacteria and fungi and also presents antitumor and antimetastatic activities. However, Biflorin is hydrophobic and photosensitive. These properties make its application difficult. In this work we prepared Biflorin micellar nanostructures looking for a more effective vehiculation and better preservation of the biological activity. Biflorin was obtained, purified and characterized by UV-Vis, infrared (IR) and 1H- and 13C-NMR. Micellar nanostructures of Biflorin were then assembled with Tween 80(R), Tween 20(R) and saline (0.9%) and characterized by UV-Vis spectroscopy and dynamic light scattering (DLS). The results showed that the micellar nanostructures were stable and presented an average size of 8.3 nm. Biflorin micellar nanostructures' photodegradation was evaluated in comparison with Biflorin in ethanol. Results showed that the Biflorin in micellar nanostructures was better protected from light than Biflorin dissolved in ethanol, and also indicated that Biflorin in micelles were efficient against Gram-positive bacteria and yeast species. In conclusion, the results showed that the micellar nanostructures could ensure the maintenance of the biological activity of Biflorin, conferring photoprotection. Moreover, Biflorin vehiculation in aqueous media was improved, favoring its applicability in biological systems.
Biflorin: an o-naphthoquinone of clinical significance.[Pubmed:25590726]
An Acad Bras Cienc. 2014 Dec;86(4):1907-14.
Biflorin is an o-naphthoquinone with proven cytotoxic effects on tumor cells showing antimicrobial, antitumor and antimutagenic activities. Biflorin is an isolated compound taken from the roots of the plant Capraria biflora L. (Schrophulariaceae), indigenous of the West Indies and South America, which is located in temperate or tropical areas. This compound has shown to be strongly active against grampositive and alcohol-acid-resistant bacteria. It has been efficient in inhibiting the proliferation tumor cell lines CEM, HL-60, B16, HCT-8 and MCF-7. Recently, SK-Br3 cell line was treated with Biflorin showing important cytotoxic effects. In this article, information related to the first structural characterization studies are presented, as well as the latest reports concerning the biological activity of this molecule.
[Study on blood enriching effects of gamma-ray radiation of paeoniflorin and albiflorin on mouse model of blood deficiency].[Pubmed:25423839]
Zhongguo Zhong Yao Za Zhi. 2014 Aug;39(15):2952-5.
OBJECTIVE: To study the blood enriching effects of Paeoniae Radix Rubra and Paeoniae Radix Alba, paeoniflorin and alBiflorin on mouse model of blood deficiency caused by gamma-ray radiation. METHOD: Build mouse model of blood deficiency induced by gamma-ray radiation. Paeoniae Radix Rubra and Paeoniae Radix Alba were given during modeling. The amount of WBC was detected af- ter the treatment. Based on the result of WBC and paeoniflorin content, alBiflorin content in Paeoniae Radix Rubra and Paeoniae Radix Alba, the same model and the same method were used to comparatively study the effect of blood enriching of paeoniflorin and alBiflorin. RESULT: On the 7th day, the amount of WBC in model mice treated with 2 g x kg(-1) Paeoniae Radix Alba and 2 g x kg(-1) Paeoniae Radix Rubra significantly increased compared with that of model group (P < 0.05). In another experiment with the same model, the amount of WBC in model mice treated with 120 mg x kg(-1) paeoflorin and 120 mg x kg(-1) alBiflorin significantly increased (P < 0.05) compared with that of model group on the 7th day. On the 10th day, the amount of WBC in rats treated with 120 mg x kg(-1) paeoflorin increased significantly (P < 0.05) compared with that of model group. Compared with the same dose of paeoniflorin, the amount of WBC in mice treated with alBiflorin had no significant difference. CONCLUSION: All Paeoniae Radix Alba, Paeoniae Radix Rubra, paeoniflorin and al- Biflorin can raise the amount of WBC and have the effect of enriching blood induced by radiation, while paeoniflorin and alBiflorin have a similar result in this model. The result indicated that both paeoniflorin and alBiflorin are effective constituents in Paeoniae Radix Alba, and paeoniflorin work as the common effective constituent in both Paeoniae Radix Rubra and Paeoniae Radix Alba.
Novel findings about management of gastric cancer: a summary from 10th IGCC.[Pubmed:25083072]
World J Gastroenterol. 2014 Jul 21;20(27):8986-92.
The Tenth International Gastric Cancer Congress (IGCC) was held in Verona, Italy, from June 19 to 22, 2013. The meeting enclosed various aspects of stomach tumor management, including both tightly clinical approaches, and topics more related to basic research. Moreover, an overview on gastrointestinal stromal tumors was provided too, although here not discussed. Here we will discuss some topics related to molecular biology of gastric cancer (GC), inherent to prognostic, diagnostic and therapeutic tools shown at the conference. Results about well known subjects, such as E-cadherin loss of expression/function, were presented. They revealed that other mutations of the gene were identified, showing a continuous research to improve diagnosis and prognosis of stomach tumor. Simultaneously, new possible molecular markers with an established role for other neoplasms, were discussed, such as mesothelin, stomatin-like protein 2 and Notch-1. Hence, a wide overview including both old and new diagnostic/prognostic tools was offered. Great attention was also dedicated to possible drugs to be used against GC. They included monoclonal antibodies, such as MS57-2.1, drugs used in other pathologies, such as maraviroc, and natural extracts from plants such as Biflorin. We would like to contribute to summarize the most impressive studies presented at the IGCC, concerning novel findings about molecular biology of gastric cancer. Although further investigations will be necessary, it can be inferred that more and more tools were developed, so as to better face stomach neoplasms.
A novel o-naphtoquinone inhibits N-cadherin expression and blocks melanoma cell invasion via AKT signaling.[Pubmed:23912027]
Toxicol In Vitro. 2013 Oct;27(7):2076-83.
The down-regulation or loss of epithelial markers is often accompanied by the up-regulation of mesenchymal markers. E-cadherin generally suppresses invasiveness, whereas N-cadherin promotes invasion and metastasis in vitro. The aim of this work is to investigate the role of Biflorin, a naphthoquinone with proven anticancer properties, on the expression of N-cadherin and AKT proteins in MDA-MB-435 invasive melanoma cancer cells after 12h of exposure to 1, 2.5 and 5 muM Biflorin. Biflorin inhibited MDA-MB-435 invasion in a dose-dependent manner (p<0.01). Likewise, Biflorin down-regulated N-cadherin and AKT-1 expression in a dose-dependent manner. Biflorin did not inhibit the adhesion of MDA-MB-435 cells to any tested substrates. Additionally, Biflorin blocked the invasiveness of cells by down-regulating N-cadherin, most likely via AKT-1 signaling. As such, Biflorin may be a novel anticancer agent and a new prototype for drug design.


