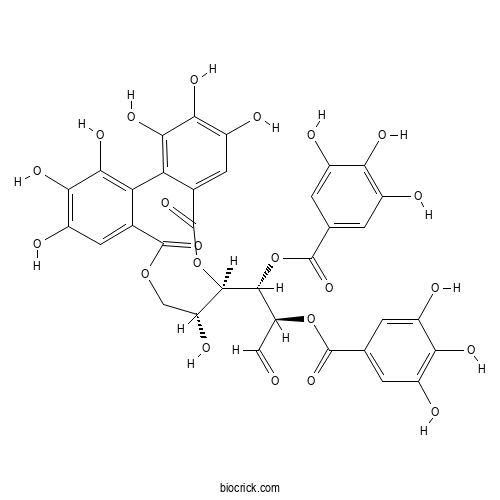
Tellimagrandin ICAS# 79786-08-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79786-08-6 | SDF | Download SDF |
| PubChem ID | 73179 | Appearance | Powder |
| Formula | C34H26O22 | M.Wt | 786.6 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,2R)-1-[(10R,11R)-3,4,5,11,17,18,19-heptahydroxy-8,14-dioxo-9,13-dioxatricyclo[13.4.0.02,7]nonadeca-1(19),2,4,6,15,17-hexaen-10-yl]-3-oxo-1-(3,4,5-trihydroxybenzoyl)oxypropan-2-yl] 3,4,5-trihydroxybenzoate | ||
| SMILES | C1C(C(OC(=O)C2=CC(=C(C(=C2C3=C(C(=C(C=C3C(=O)O1)O)O)O)O)O)O)C(C(C=O)OC(=O)C4=CC(=C(C(=C4)O)O)O)OC(=O)C5=CC(=C(C(=C5)O)O)O)O | ||
| Standard InChIKey | XUZYVFYOPRXTRB-GBJTXXJHSA-N | ||
| Standard InChI | InChI=1S/C34H26O22/c35-7-20(54-31(49)9-1-13(36)23(43)14(37)2-9)30(56-32(50)10-3-15(38)24(44)16(39)4-10)29-19(42)8-53-33(51)11-5-17(40)25(45)27(47)21(11)22-12(34(52)55-29)6-18(41)26(46)28(22)48/h1-7,19-20,29-30,36-48H,8H2/t19-,20+,29-,30-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tellimagrandin I Dilution Calculator

Tellimagrandin I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2713 mL | 6.3565 mL | 12.7129 mL | 25.4259 mL | 31.7824 mL |
| 5 mM | 0.2543 mL | 1.2713 mL | 2.5426 mL | 5.0852 mL | 6.3565 mL |
| 10 mM | 0.1271 mL | 0.6356 mL | 1.2713 mL | 2.5426 mL | 3.1782 mL |
| 50 mM | 0.0254 mL | 0.1271 mL | 0.2543 mL | 0.5085 mL | 0.6356 mL |
| 100 mM | 0.0127 mL | 0.0636 mL | 0.1271 mL | 0.2543 mL | 0.3178 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- O-Methylglycosolone
Catalog No.:BCX2112
CAS No.:41303-25-7
- Garcinexanthone C
Catalog No.:BCX2111
CAS No.:1107620-69-8
- Mangostenone F
Catalog No.:BCX2110
CAS No.:1242438-70-5
- cis-N-Terrestriamide
Catalog No.:BCX2109
CAS No.:2170469-93-7
- Morindaparvin W
Catalog No.:BCX2108
CAS No.:2596365-67-0
- 25(27)-Ene-elephanoside H
Catalog No.:BCX2107
CAS No.:1592406-40-0
- Flindersine
Catalog No.:BCX2106
CAS No.:523-64-8
- Isorhamnetin 3-O-apiosyl (1->2)[rhamnosyl (1->6)]glucoside
Catalog No.:BCX2105
CAS No.:165605-18-5
- 25R-Tupistroside E
Catalog No.:BCX2104
CAS No.:1448639-95-9
- Stellarin 2
Catalog No.:BCX2103
CAS No.:63975-58-6
- 3',4'-Dimethoxytaxifolin
Catalog No.:BCX2102
CAS No.:179871-73-9
- Kaempferol 3-O-malonylglucoside
Catalog No.:BCX2101
CAS No.:81202-52-0
- 3alpha-Hinokiol
Catalog No.:BCX2114
CAS No.:107740-34-1
- Biflorin
Catalog No.:BCX2115
CAS No.:89701-85-9
- 6-Hydroxy-2-methoxyacetophenone 4-O-beta-D-xylopyranosyl-(1->6)-beta-D-glucopyranoside
Catalog No.:BCX2116
CAS No.:2140317-59-3
- Fischeroside B
Catalog No.:BCX2117
CAS No.:1307257-08-4
- Isolophanthin B
Catalog No.:BCX2118
CAS No.:1370511-57-1
- 7-O-Methylhorminone
Catalog No.:BCX2119
CAS No.:122482-10-4
- Isocarthamidin 7-O-glucuronide
Catalog No.:BCX2120
CAS No.:119600-61-2
- 6,8-Di-C-beta-D-glucopyranosylacacetin
Catalog No.:BCX2121
CAS No.:28234-99-3
- 1,5-Epoxy-3S-hydroxy-1-(3,4-dihydroxy-5-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane
Catalog No.:BCX2122
CAS No.:182369-54-6
- Callicapoic acid M1
Catalog No.:BCX2123
CAS No.:2756970-65-5
- 8-C-beta-D-(6-O-galloyl)glucosylnoreugenin
Catalog No.:BCX2124
CAS No.:152041-17-3
- 3'-Methoxynyasin
Catalog No.:BCX2125
CAS No.:1685246-20-1
Copper(II)-Amine Complex-Mediated Intramolecular Coupling of Gallates: A Bioinspired Solution to the Atroposelective Synthesis of Ellagitannins.[Pubmed:39254887]
Angew Chem Int Ed Engl. 2024 Dec 20;63(52):e202412036.
Total syntheses of the C-glucosidic ellagitannins (-)-punicacortein A, (-)-epipunicacortein A and (+)-castalin were accomplished for the first time, and those of the glucopyranosic ellagitannins (+)-Tellimagrandin I and (+)-pedunculagin were revisited. The atroposelective construction of their characteristic hexahydroxydiphenoyl (HHDP) and nonahydroxyterphenoyl (NHTP) units relied on the use of different cupric-amine complexes under different reaction conditions to mediate the intramolecular dehydrogenative coupling of galloyl groups at different positions of glucose cores. In particular, the monodentate n-butylamine and the bidentate (-)-sparteine were found to be complementary in their capacity to promote the regio- and atroposelective coupling of galloyl groups on a (4)C(1)-glucopyranosic core into 2,3-O-(S)- and/or 4,6-O-(S)-HHDP units. Furthermore, replacing (-)-sparteine by its optical antipode not only counteracted the substrate-controlled induction of atroposelectivity to forge a 4,6-O-(R)-HHDP unit, but it also enabled a (4)C(1) to (1)C(4) ring flip of the glucopyranosic core and hence the formation of 2,4-O-(R)- and 3,6-O-(R)-HHDP units, such as those featured in the glucopyranosic ellagitannins phyllanemblinin B and geraniin.
Rosa rugosa Low Caloric Fiber Protein Preparations Rich in Antioxidant Flavanols and Ellagitannins.[Pubmed:38138511]
Molecules. 2023 Dec 9;28(24):8021.
Defatted seed residues after the extraction of rose oil have their potential not fully described in the existing literature. The aim of this study was to determine and characterize the components important for the human body that are found in Rosa rugosa defatted seeds, including dietary fibers, proteins, selected minerals, polyphenols and antioxidant activity. Rosa rugosa seeds defatted with CO(2) in supercritical conditions are a rich source of dietary fibers (approx. 65%) and proteins (15%); their macronutrients include the following: Ca (175.9), Mg (83.9), K (199.2) and Na (3.5 mg/100 g). They also contain polyphenols, including flavanols (0.9%) and total ellagic acid (0.5%), and they exhibit antioxidant activity (143.8 microM TAEC/g). Tellimagrandin I and II and rugosin A were found in the extracts, and ellagitannins with a yet-indeterminate structure were also present. The seeds also contained ellagitannin derivatives-galloyl-HHDP-glucose and bis-HHDP-glucose-at the same time, and they are characterized by a low-fat content-0.4%. The energy value of defatted rose seeds is about half the energy value of popular seeds used in the food industry. The findings of the present study suggest that defatted rosehip seeds, the by-product of rosehip processing, could be an important source of bioactive components like dietary fibers, flavanols, ellagitannins and mineral compounds. Therefore, defatted rose seeds are very promising and require further research, because they can potentially be used as a natural source of chemopreventive agents.
The potential antidiabetic properties of green and purple tea [Camellia sinensis (L.) O Kuntze], purple tea ellagitannins, and urolithins.[Pubmed:36907477]
J Ethnopharmacol. 2023 Jun 12;309:116377.
ETHNOPHARMACOLOGICAL RELEVANCE: Tea (Camellia sinensis) has been consumed for centuries as traditional medicine for various diseases, including diabetes. The mechanism of action of many traditional medicines, including tea, often requires elucidation. Purple tea is a natural mutant of Camellia sinensis, grown in China and Kenya, and is rich in anthocyanins and ellagitannins. AIM OF THE STUDY: Here we aimed to determine whether commercial green and purple teas are a source of ellagitannins and whether green and purple teas, purple tea ellagitannins and their metabolites urolithins have antidiabetic activity. MATERIALS AND METHODS: Targeted UPLC-MS/MS was employed to quantify the ellagitannins corilagin, strictinin and Tellimagrandin I, in commercial teas. The inhibitory effect of commercial green and purple teas and purple tea ellagitannins was evaluated on alpha-glucosidase and alpha-amylase. The bioavailable urolithins were then investigated for additional antidiabetic effects, by evaluating their effect on cellular glucose uptake and lipid accumulation. RESULTS: Corilagin, strictinin and Tellimagrandin I (ellagitannins) were identified as potent inhibitors of alpha-amylase and alpha-glucosidase, with K(i) values significantly lower (p < 0.05) than acarbose. Commercial green-purple teas were identified as ellagitannin sources, with especially high concentrations of corilagin. These commercial purple teas, containing ellagitannins, were identified as potent alpha-glucosidase inhibitors with IC(50) values significantly lower (p < 0.05) than green teas and acarbose. Urolithin A and urolithin B were as effective (p> 0.05) as metformin in increasing glucose uptake in adipocytes, muscle cells and hepatocytes. In addition, similar (p > 0.05) to metformin, both urolithin A and urolithin B reduced lipid accumulation in adipocytes and hepatocytes. CONCLUSIONS: This study identified green-purple teas as an affordable widely available natural source with antidiabetic properties. Furthermore, additional antidiabetic effects of purple tea ellagitannins (corilagin, strictinin and Tellimagrandin I) and urolithins were identified.
Inhibitory Effects of Hydrolysable Tannins on Lipid Accumulation in 3T3-L1 Cells.[Pubmed:36184503]
Biol Pharm Bull. 2022;45(10):1458-1465.
Obesity is currently the most common cause of metabolic diseases including type 2 diabetes and hyperlipidemia. Obesity results from excess lipid accumulation in adipose tissue. Several studies have investigated the inhibitory effects of natural plant-derived products on adipocyte differentiation and lipid accumulation. In this study, we examined the effect of hydrolysable tannins composed of gallic acid and glucose on adipocyte differentiation in 3T3-L1 cells. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose (PGG) (1), a representative gallotannin, inhibited lipid accumulation in 3T3-L1 cells, whereas ellagitannins (Tellimagrandin I, eugeniin and casuarictin) did not. The expression of adipocyte differentiation-related genes, including peroxisome proliferator activator gamma2 (Ppargamma2), CCAAT/enhancer binding protein alpha (C/EBPalpha) and adipocyte fatty acid binding protein (aP2), was significantly suppressed in PGG (1)-treated 3T3-L1 cells beginning at day 2 after induction of differentiation. While PGG (1) did not directly reduce Ppargamma2 expression, it reduced the expression of its target genes in mature adipocytes. In addition, PGG (1) treatment inhibited mitotic clonal expansion, one of earliest events of adipocyte differentiation. These findings indicate that PGG (1) has an inhibitory effect on adipocyte differentiation through the suppression of mitotic clonal expansion.
Walnut Prevents Cognitive Impairment by Regulating the Synaptic and Mitochondrial Dysfunction via JNK Signaling and Apoptosis Pathway in High-Fat Diet-Induced C57BL/6 Mice.[Pubmed:36014555]
Molecules. 2022 Aug 20;27(16):5316.
This study was conducted to evaluate the protective effect of Juglans regia (walnut, Gimcheon 1ho cultivar, GC) on high-fat diet (HFD)-induced cognitive dysfunction in C57BL/6 mice. The main physiological compounds of GC were identified as pedunculagin/casuariin isomer, strictinin, Tellimagrandin I, ellagic acid-O-pentoside, and ellagic acid were identified using UPLC Q-TOF/MS analysis. To evaluate the neuro-protective effect of GC, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2',7'-dichlorodihydrofluorecein diacetate (DCF-DA) analysis were conducted in H(2)O(2) and high glucose-induced neuronal PC12 cells and hippocampal HT22 cells. GC presented significant cell viability and inhibition of reactive oxygen species (ROS) production. GC ameliorated behavioral and memory dysfunction through Y-maze, passive avoidance, and Morris water maze tests. In addition, GC reduced white adipose tissue (WAT), liver fat mass, and serum dyslipidemia. To assess the inhibitory effect of antioxidant system deficit, lipid peroxidation, ferric reducing antioxidant power (FRAP), and advanced glycation end products (AGEs) were conducted. Administration of GC protected the antioxidant damage against HFD-induced diabetic oxidative stress. To estimate the ameliorating effect of GC, acetylcholine (ACh) level, acetylcholinesterase (AChE) activity, and expression of AChE and choline acetyltransferase (ChAT) were conducted, and the supplements of GC suppressed the cholinergic system impairment. Furthermore, GC restored mitochondrial dysfunction by regulating the mitochondrial ROS production and mitochondrial membrane potential (MMP) levels in cerebral tissues. Finally, GC ameliorated cerebral damage by synergically regulating the protein expression of the JNK signaling and apoptosis pathway. These findings suggest that GC could provide a potential functional food source to improve diabetic cognitive deficits and neuronal impairments.
Hydrolyzable tannins (ellagitannins), flavonoids, pentacyclic triterpenes and their glycosides in antimycobacterial extracts of the ethnopharmacologically selected Sudanese medicinal plant Combretum hartmannianum Schweinf.[Pubmed:34624680]
Biomed Pharmacother. 2021 Dec;144:112264.
In Sudanese traditional medicine, decoctions, macerations, and tonics of the stem and root of Combretum hartmannianum are used for the treatment of persistent cough, a symptom that could be related to tuberculosis (TB). To verify these traditional uses, extracts from the stem wood, stem bark, and roots of C. hartmannianum were screened for their growth inhibitory effects against Mycobacterium smegmatis ATCC 14468. Methanol Soxhlet and ethyl acetate extracts of the root gave the strongest effects (MIC 312.5 and 625 microg/ml, respectively). HPLC-UV/DAD and UHPLC/QTOF-MS analysis of the ethyl acetate extract of the root led to the detection of 54 compounds, of which most were polyphenols and many characterized for the first time in C. hartmannianum. Among the major compounds were terflavin B and its two isomers, castalagin, corilagin, Tellimagrandin I and its derivative, (S)-flavogallonic acid dilactone, punicalagin, and methyl-ellagic acid xylopyranoside. In addition, di-, tri- and tetra-galloyl glucose, combregenin, terminolic acid, cordifoliside D, luteolin, and quercetin-3-O-galactoside-7-O-rhamnoside-(2-->1)-O-beta-D-arabinopyranoside were characterized. Luteolin gave better growth inhibition against M. smegmatis (MIC 250 microg/ml) than corilagin, ellagic acid, and gallic acid (MIC 500-1000 microg/ml). Our study justifies the use of C. hartmannianum in Sudanese folk medicine against prolonged cough that could be related to TB infection. This study demonstrates that C. hartmannianum should be explored further for new anti-TB drug scaffolds and antibiotic adjuvants.
Pharmacological investigation of antioxidant and anti-inflammatory activities of leaves and branches extracts from Plinia cauliflora (Jaboticaba).[Pubmed:34352328]
J Ethnopharmacol. 2021 Nov 15;280:114463.
ETHNOPHARMACOLOGICAL RELEVANCE: Among all native Brazilian plant species, Plinia cauliflora (DC.) Kausel (Jaboticaba), is well known for producing "superfruits", due to their high phenolic content and antioxidant property. The fruit has astringent characteristics, and it is popularly known for the treatment of diarrhea, rash, and intestinal inflammation. However, there are only a few studies on the use of leaves and branches of this species in the literature, mainly to treat oxidative stress and inflammation. AIM OF THE STUDY: The present study aimed to investigate the antioxidant and anti-inflammatory potential of leaves and branches extracts from P. cauliflora. MATERIAL AND METHODS: The phytochemical analysis of P. cauliflora extracts was performed by the total phenolic, flavonoid, and tannin dosage method. Moreover, the compounds were identified by HPLC-MS-Q-TOF. Antioxidant capacity was determined by DPPH, beta-carotene/linoleic acid system, MDA formation, and phosphomolybdenum assays. In vitro and in vivo anti-inflammatory activities of P. cauliflora were evaluated by the reduction of nitric oxide in the J774A.1 cell line and inhibition of ear edema in mice, respectively. RESULTS: The ethanolic extract of the leaves exhibited greater flavonoid content whereas the ethanolic extract of the branches showed higher tannins content. Twenty-two and seventeen compounds were identified by HPLC-MS-Q-TOF in the leaves and branches, respectively, being Tellimagrandin I, castalagin, and valoneic acid dilactone reported for the first time in P. cauliflora. The antioxidant potential of extracts was confirmed through different oxidation pathways from oxidizing radicals, which might be related to the presence of phenolic compounds. For the anti-inflammatory assay, the leaves and branches extracts showed promising results, with a reduction of nitric oxide ear edema inhibition around 95% and 80%, respectively. CONCLUSIONS: Herein, the great biological potential of leaves and branches extracts from P. cauliflora was highlighted. These parts of the plant are underused and poorly reported in the literature, especially for the antioxidant and anti-inflammatory activities.
Barricyclin D1-a dimeric ellagitannin with a macrocyclic structure-and accompanying tannins from Barringtonia racemosa.[Pubmed:33890626]
Biosci Biotechnol Biochem. 2021 Jun 24;85(7):1609-1620.
Our examination of high molecular weight polyphenolic constituents in the leaves of Barringtonia racemosa of the family Lecythidaceae uncovered 5 previously undescribed ellagitannins. One, barringtin M1 (1), among them was a hydrolysable tannin monomer, while remaining 4, barringtins D1 (2), D2 (3), D3 (4), and barricyclin D1 (5), were all dimers. Barricyclin D1 had a first macrocyclic structure formed from casuarictin (6) and Tellimagrandin I (7), and the other ellagitannins had structures related to 5. Two additional known phenolics, valoneic acid dilactone (8) and schimawalin A (9), were also isolated from the leaves. These results suggested that the leaves of B. racemosa are a natural resource rich in hydrolysable tannin oligomers.
Discovering Potential RNA Dependent RNA Polymerase Inhibitors as Prospective Drugs Against COVID-19: An in silico Approach.[Pubmed:33716752]
Front Pharmacol. 2021 Feb 26;12:634047.
COVID-19, caused by Severe Acute Respiratory Syndrome Corona Virus 2, is declared a Global Pandemic by WHO in early 2020. In the present situation, though more than 180 vaccine candidates with some already approved for emergency use, are currently in development against SARS-CoV-2, their safety and efficacy data is still in a very preliminary stage to recognize them as a new treatment, which demands an utmost emergency for the development of an alternative anti-COVID-19 drug sine qua non for a COVID-19 free world. Since RNA-dependent RNA polymerase (RdRp) is an essential protein involved in replicating the virus, it can be held as a potential drug target. We were keen to explore the plant-based product against RdRp and analyze its inhibitory potential to treat COVID-19. A unique collection of 248 plant compounds were selected based on their antiviral activity published in previous literature and were subjected to molecular docking analysis against the catalytic sub-unit of RdRp. The docking study was followed by a pharmacokinetics analysis and molecular dynamics simulation study of the selected best-docked compounds. Tellimagrandin I, SaikosaponinB2, Hesperidin and (-)-Epigallocatechin Gallate were the most prominent ones that showed strong binding affinity toward RdRp. All the compounds mentioned showed satisfactory pharmacokinetics properties and remained stabilized at their respective binding sites during the Molecular dynamics simulation. Additionally, we calculated the free-binding energy/the binding properties of RdRp-ligand complexes with the connection of MM/GBSA. Interestingly, we observe that SaikosaponinB2 gives the best binding affinity (∆G(binding) = -42.43 kcal/mol) in the MM/GBSA assay. Whereas, least activity is observed for Hesperidin (∆G(binding) = -22.72 kcal/mol). Overall our study unveiled the feasibility of the SaikosaponinB2 to serve as potential molecules for developing an effective therapy against COVID-19 by inhibiting one of its most crucial replication proteins, RdRp.
Synthesis and Comparative Structure-Activity Study of Carbohydrate-Based Phenolic Compounds as alpha-Glucosidase Inhibitors and Antioxidants.[Pubmed:31783621]
Molecules. 2019 Nov 27;24(23):4340.
Twenty-one natural and unnatural phenolic compounds containing a carbohydrate moiety were synthesized and their structure-activity relationship (SAR) was evaluated for alpha-glucosidase inhibition and antioxidative activity. Varying the position of the galloyl unit on the 1,5-anhydro-d-glucitol (1,5-AG) core resulted in changes in the alpha-glucosidase inhibitory activity and notably, particularly strong activity was demonstrated when the galloyl unit was present at the C-2 position. Furthermore, increasing the number of the galloyl units significantly affected the alpha-glucosidase inhibition, and 2,3,4,6-tetra-galloyl-1,5-AG (54) and 2,3,4,6-tetra-galloyl-d-glucopyranose (61) exhibited excellent activities, which were more than 13-fold higher than the alpha-glucosidase inhibitory activity of acertannin (37). Moreover, a comparative structure-activity study suggested that a hemiacetal hydroxyl functionality in the carbohydrate core and a biaryl bond of the 4,6-O-hexahydroxydiphenoyl (HHDP) group, which are components of ellagitannins including Tellimagrandin I, are not necessary for the alpha-glucosidase inhibitory activity. Lastly, the antioxidant activity increased proportionally with the number of galloyl units.
Anti-acne vulgaris effect including skin barrier improvement and 5alpha-reductase inhibition by tellimagrandin I from Carpinus tschonoskii.[Pubmed:31752827]
BMC Complement Altern Med. 2019 Nov 21;19(1):323.
BACKGROUND: Carpinus tschonoskii (CT) has been previously studied for various activities in the improvement of skin diseases. In the present study, we examined the in vitro anti-acne vulgaris (AV) effect of CT leaves (CTL) and Tellimagrandin I (TI), one of the main ellagitannins from CT, including skin barrier improvement and 5alpha-reductase inhibitory activity. METHODS: To test the anti-AV activities of CTL and TI, firstly, anti-oxidative and anti-inflammatory activities including DPPH radical scavenging activity, nitric oxide (NO) inhibitory activity, and cytokines [interleukin (IL)-6 and IL-8] were tested. Skin barrier improvement experiments were tested using developing cornified envelope (CE) formation, and filaggrin mRNA expression level was determined by RT-PCR. The 5alpha-reductase inhibitory activity was determined by measuring the testosterone levels in rat liver microsomes. RESULTS: CTL and TI showed potent anti-oxidative activity and anti-inflammatory activities. Especially, the cytokine production inhibitory activities of TI were found to be similar to the positive control, epigallocatechin gallate (EGCG). CTL and TI enhanced the CE formation and filaggrin mRNA expression levels and showed potent activities compared to that in the positive control, 1.5 mM Ca(2+). In additionally, CTL and TI showed 5alpha-reductase inhibitory activities in a dose-dependent manner. CONCLUSION: The results showed that CTL and TI inhibit AV endogenous factors such as 5alpha-reductase and inflammatory cytokines and affect exogenous factors such as developing skin barrier function (CE and filaggrin levels). Therefore, CTL and TI may be plant-derived agent, promising in the treatment of acne vulgaris.
Inhibition of protein phosphatase-1 and -2A by ellagitannins: structure-inhibitory potency relationships and influences on cellular systems.[Pubmed:30696301]
J Enzyme Inhib Med Chem. 2019 Dec;34(1):500-509.
Several ellagitannins inhibited the activity of protein phosphatase-1 (PP1) and -2 A (PP2A) catalytic subunits (PP1c and PP2Ac) with preferential suppression of PP1c over PP2Ac. The inhibitory potency for PP1c followed the order of Tellimagrandin I > mahtabin A > praecoxin B > 1.2-Di-O-galloyl-4.6-(S)-HHDP-beta-D-glucopyranose > pedunculagin with IC(50) values ranging from 0.20 microM to 2.47 microM. The interaction of PP1c and Tellimagrandin I was assessed by NMR saturation transfer difference, surface plasmon resonance, isothermal titration calorimetry, and microscale thermophoresis based binding techniques. Tellimagrandin I suppressed viability and phosphatase activity of HeLa cells, while mahtabin A was without effect. Conversely, mahtabin A increased the phosphorylation level of SNAP-25(Thr138) and suppressed exocytosis of cortical synaptosomes, whereas Tellimagrandin I was without influence. Our results establish ellagitannins as partially selective inhibitors of PP1 and indicate that these polyphenols may act distinctly in cellular systems depending on their membrane permeability and/or their actions on cell membranes.


