Bis(2-ethylhexyl) terephthalateCAS# 6422-86-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6422-86-2 | SDF | Download SDF |
| PubChem ID | 22932 | Appearance | Oil |
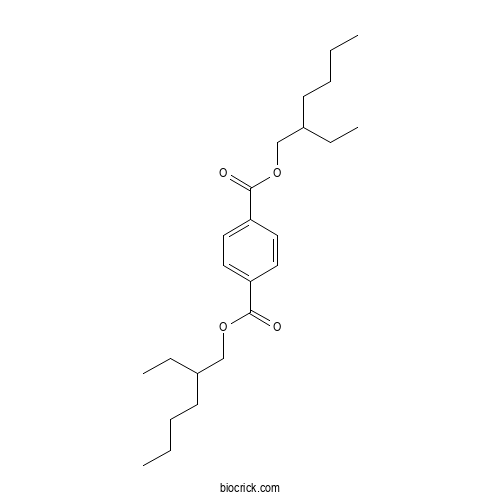
| Formula | C24H38O4 | M.Wt | 390.6 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | bis(2-ethylhexyl) benzene-1,4-dicarboxylate | ||
| SMILES | CCCCC(CC)COC(=O)C1=CC=C(C=C1)C(=O)OCC(CC)CCCC | ||
| Standard InChIKey | RWPICVVBGZBXNA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H38O4/c1-5-9-11-19(7-3)17-27-23(25)21-13-15-22(16-14-21)24(26)28-18-20(8-4)12-10-6-2/h13-16,19-20H,5-12,17-18H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bis(2-ethylhexyl) terephthalate Dilution Calculator

Bis(2-ethylhexyl) terephthalate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5602 mL | 12.8008 mL | 25.6016 mL | 51.2033 mL | 64.0041 mL |
| 5 mM | 0.512 mL | 2.5602 mL | 5.1203 mL | 10.2407 mL | 12.8008 mL |
| 10 mM | 0.256 mL | 1.2801 mL | 2.5602 mL | 5.1203 mL | 6.4004 mL |
| 50 mM | 0.0512 mL | 0.256 mL | 0.512 mL | 1.0241 mL | 1.2801 mL |
| 100 mM | 0.0256 mL | 0.128 mL | 0.256 mL | 0.512 mL | 0.64 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Toxyloxanthone B
Catalog No.:BCN0456
CAS No.:50875-24-6
- V1 iridoid
Catalog No.:BCN0455
CAS No.:87441-71-2
- Acronyculatin Q
Catalog No.:BCN0454
CAS No.:2287183-54-2
- Mecambridine
Catalog No.:BCN0453
CAS No.:31098-60-9
- Acropyrone
Catalog No.:BCN0452
CAS No.:1381875-14-4
- Furanofukinin
Catalog No.:BCN0451
CAS No.:34335-93-8
- 6-O-Ethyltetradymodiol
Catalog No.:BCN0450
CAS No.:1028530-06-4
- 6β-Ethoxyfuranoeremophilane
Catalog No.:BCN0449
CAS No.:82462-36-0
- Phenylacetylglycine
Catalog No.:BCN0448
CAS No.:500-98-1
- N1-Methyl-2-pyridone-5-carboxamide
Catalog No.:BCN0447
CAS No.:701-44-0
- Petasalbin
Catalog No.:BCN0446
CAS No.:4176-11-8
- β-D-Allose
Catalog No.:BCN0445
CAS No.:2595-97-3
- (+/-)-N-methylcoclaurine
Catalog No.:BCN0458
CAS No.:1472-62-4
- 5-exo-Hydroxyborneol
Catalog No.:BCN0459
CAS No.:32751-73-8
- 3,8-Di-O-methylellagic acid 2-O-(5'-O-acetyl)arabinofuranoside
Catalog No.:BCN0460
CAS No.:365540-09-6
- 4,5-Dioxodehydroasimilobine
Catalog No.:BCN0461
CAS No.:82644-81-3
- 2,4-Dihydroxy-6-methoxy-3,5-diprenylacetophenone
Catalog No.:BCN0462
CAS No.:123999-38-2
- Alpinigenine
Catalog No.:BCN0463
CAS No.:14028-91-2
- Tabernaecorymbosine A
Catalog No.:BCN0464
CAS No.:1262306-81-9
- Flindulatin
Catalog No.:BCN0465
CAS No.:521-44-8
- (±)-Cleroindicin F
Catalog No.:BCN0466
CAS No.:94535-01-0
- p-O-Farnesylcoumaric acid
Catalog No.:BCN0467
CAS No.:939769-47-8
- 6,4'-Dihydroxy-5,7-dimethoxyflavanone
Catalog No.:BCN0468
CAS No.:6951-57-1
- 5-endo-Hydroxyborneol
Catalog No.:BCN0469
CAS No.:68738-10-3
A practical approach for the determination of environmental quality standards-based discharge limits: the case of Tersakan sub-basin of Yesilirmak River in Turkey.[Pubmed:33742382]
Environ Sci Pollut Res Int. 2021 Aug;28(29):38730-38748.
The control of point source discharges to rivers has become more significant since the establishment of environmental quality standards (EQSs). Many countries, including Turkey, have set EQS values for various contaminants. One important challenge regarding these EQSs is to reconcile the effluent limits that are technically and economically achievable with the ones that are required to accomplish the EQSs. The Tersakan sub-basin of Yesilirmak River acquires good examples of this challenge due to the industrial and agricultural discharge activities present. In this study, a new, simplistic, and less data-driven approach is developed to facilitate this compromise and implemented for all suitable discharge points within the sub-basin. The foundation of this approach is that effluent discharges may mix and become diluted within negligibly short distances from the point of discharge where exceedance of EQSs can be permissible. The approach modularly combines different analytical solutions of the advective-dispersive mass transport equation that are applicable under different mixing conditions and estimates maximum allowable discharge concentrations of contaminants. The results of the case study in the Tersakan sub-basinindicate that none of the studied discharges need load reduction to achieve EQSs. However, in various points, tridecane, nickel, Bis(2-ethylhexyl) terephthalate, NH4-N, total phosphorus, and free cyanide have consumed more than 10% of their discharge quotas estimated by the mentioned approach. Therefore, for the sub-basin, these six contaminants and their corresponding two discharge points may require more attention in the future.
Recent Attempts in the Design of Efficient PVC Plasticizers with Reduced Migration.[Pubmed:33578880]
Materials (Basel). 2021 Feb 10;14(4). pii: ma14040844.
This paper reviews the current trends in replacing commonly used plasticizers in poly(vinyl chloride), PVC, formulations by new compounds with reduced migration, leading to the enhancement in mechanical properties and better plasticizing efficiency. Novel plasticizers have been divided into three groups depending on the replacement strategy, i.e., total replacement, partial replacement, and internal plasticizers. Chemical and physical properties of PVC formulations containing a wide range of plasticizers have been compared, allowing observance of the improvements in polymer performance in comparison to PVC plasticized with conventionally applied bis(2-ethylhexyl) phthalate, di-n-octyl phthalate, Bis(2-ethylhexyl) terephthalate and di-n-octyl terephthalate. Among a variety of newly developed plasticizers, we have indicated those presenting excellent migration resistance and advantageous mechanical properties, as well as those derived from natural sources. A separate chapter has been dedicated to the description of a synergistic effect of a mixture of two plasticizers, primary and secondary, that benefits in migration suppression when secondary plasticizer is added to PVC blend.
Dietary Exposure Estimation to Chemicals Transferred from Milk and Dairy Products Packaging Materials in Spanish Child and Adolescent Population.[Pubmed:33121003]
Foods. 2020 Oct 27;9(11). pii: foods9111554.
Packaging materials are subject to risk assessment since they can transfer their components to the food, and they may constitute a risk for the consumers' health. Therefore, estimating the exposure to chemicals migrating from packaging is required. In this study, a novel approach based on a total diet study (TDS)-like investigation to evaluate the exposure to chemicals transferred from the packaging was presented. The proposed methodology involved a non-targeted gas chromatography coupled to mass spectrometry (GC-MS) method to identify potential migrants and the determination of the migrants in composite food samples. The method was applied to evaluate the dietary exposure to chemicals from food packaging materials used for milk and dairy products in the Spanish child and adolescent populations. Several migrants identified in packaging materials were selected to determine their concentration in composite food samples. These chemicals included diethyl phthalate (DEP), diisobutyl phthalate (DIBP), dibutyl phthalate (DBP), bis(2ethylhexyl) phthalate (DEHP), benzophenone (BP), 1,3-diphenylpropane (1,3-DPP), and Bis(2-ethylhexyl) terephthalate (DEHT). The method exhibited a good sensitivity (limit of detection, LOD
Children's exposure to phthalates and non-phthalate plasticizers in the home: The TESIE study.[Pubmed:31400598]
Environ Int. 2019 Nov;132:105061.
BACKGROUND: Phthalates and their potential replacements, including non-phthalate plasticizers, are ubiquitous in home environments due to their presence in building materials, plastics, and personal care products. As a result, exposure to these compounds is universal. However, the primary pathways of exposure and understanding which products in the home are associated most strongly with particular exposures are unclear. OBJECTIVES: We sought to investigate the relationships between phthalates and non-phthalate plasticizers in paired samples of house dust, hand wipes, and their corresponding metabolites in children's urine samples (n=180). In addition, we compared product use or presence of materials in the household against all compounds to investigate the relationship between product use or presence and exposure. METHODS: Children aged 3-6years provided hand wipe and urine samples. Questionnaires were completed by mothers or legal guardians to capture product use and housing characteristics, and house dust samples were collected from the main living area during home visits. RESULTS: Phthalates and non-phthalate replacements were detected frequently in the environmental matrices. All urine samples had at least 13 of 19 phthalate or non-phthalate replacement metabolites present. Hand wipe mass and dust concentrations of diisobutyl phthalate, benzyl butyl phthalate (BBP), bis(2-ethylhexyl) phthalate, and di-isononyl phthalate were significantly associated with their corresponding urinary metabolites (rs=0.18-0.56, p<0.05). Bis(2-ethylhexyl) terephthalate (DEHTP) in dust was also significantly and positively correlated with its urinary metabolites (rs=0.33, p<0.001). Vinyl flooring was most significantly and positively associated with particular phthalate exposures (indicated by concentrations in environmental matrices and urinary biomarkers). In particular, children who lived in homes with 100% vinyl flooring had urinary concentrations of monobenzyl phthalate, a BBP metabolite, that were 15 times higher than those of children who lived in homes with no vinyl flooring (p<0.0001). Levels of BBP in hand wipes and dust were 3.5 and 4.5 times higher, respectively, in those homes with 100% vinyl flooring (p<0.0001 for both). CONCLUSIONS: This paper summarizes one of the most comprehensive phthalate and non-phthalate plasticizer investigation of potential residential exposure sources conducted in North America to date. The data presented herein provide evidence that dermal contact and hand-to-mouth behaviors are important sources of exposure to phthalates and non-phthalate plasticizers. In addition, the percentage of vinyl flooring is an important consideration when examining residential exposures to these compounds.


