PhenylacetylglycineCAS# 500-98-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 500-98-1 | SDF | Download SDF |
| PubChem ID | 68144 | Appearance | Powder |
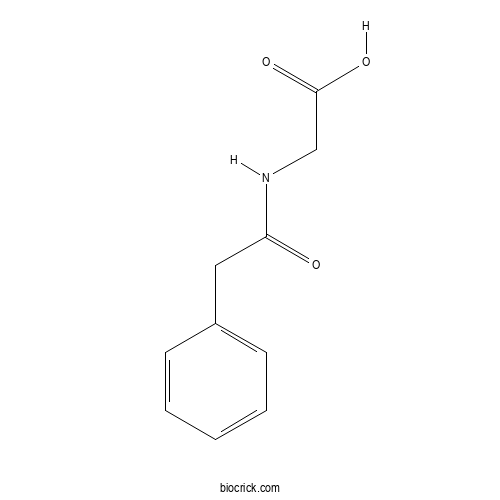
| Formula | C10H11NO3 | M.Wt | 193.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Phenaceturic acid | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[(2-phenylacetyl)amino]acetic acid | ||
| SMILES | C1=CC=C(C=C1)CC(=O)NCC(=O)O | ||
| Standard InChIKey | UTYVDVLMYQPLQB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H11NO3/c12-9(11-7-10(13)14)6-8-4-2-1-3-5-8/h1-5H,6-7H2,(H,11,12)(H,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Phenylacetylglycine Dilution Calculator

Phenylacetylglycine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.176 mL | 25.8799 mL | 51.7598 mL | 103.5197 mL | 129.3996 mL |
| 5 mM | 1.0352 mL | 5.176 mL | 10.352 mL | 20.7039 mL | 25.8799 mL |
| 10 mM | 0.5176 mL | 2.588 mL | 5.176 mL | 10.352 mL | 12.94 mL |
| 50 mM | 0.1035 mL | 0.5176 mL | 1.0352 mL | 2.0704 mL | 2.588 mL |
| 100 mM | 0.0518 mL | 0.2588 mL | 0.5176 mL | 1.0352 mL | 1.294 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N1-Methyl-2-pyridone-5-carboxamide
Catalog No.:BCN0447
CAS No.:701-44-0
- Petasalbin
Catalog No.:BCN0446
CAS No.:4176-11-8
- β-D-Allose
Catalog No.:BCN0445
CAS No.:2595-97-3
- Alaschanioside A
Catalog No.:BCN0444
CAS No.:157446-63-4
- 19-Epivoacristine
Catalog No.:BCN0443
CAS No.:6883-77-8
- Borneol glucuronide
Catalog No.:BCN0442
CAS No.:1642562-41-1
- Acrovestenol
Catalog No.:BCN0441
CAS No.:864679-69-6
- Farfugin A
Catalog No.:BCN0440
CAS No.:36061-18-4
- Cyclobutanedichalcone
Catalog No.:BCN0439
CAS No.:861706-38-9
- Genipin 10-O-glucoside
Catalog No.:BCN0438
CAS No.:1947317-95-4
- 19-Hydroxypseudovincadifformine
Catalog No.:BCN0437
CAS No.:2340171-73-3
- Tuberostemonine D
Catalog No.:BCN0436
CAS No.:1627827-84-2
- 6β-Ethoxyfuranoeremophilane
Catalog No.:BCN0449
CAS No.:82462-36-0
- 6-O-Ethyltetradymodiol
Catalog No.:BCN0450
CAS No.:1028530-06-4
- Furanofukinin
Catalog No.:BCN0451
CAS No.:34335-93-8
- Acropyrone
Catalog No.:BCN0452
CAS No.:1381875-14-4
- Mecambridine
Catalog No.:BCN0453
CAS No.:31098-60-9
- Acronyculatin Q
Catalog No.:BCN0454
CAS No.:2287183-54-2
- V1 iridoid
Catalog No.:BCN0455
CAS No.:87441-71-2
- Toxyloxanthone B
Catalog No.:BCN0456
CAS No.:50875-24-6
- Bis(2-ethylhexyl) terephthalate
Catalog No.:BCN0457
CAS No.:6422-86-2
- (+/-)-N-methylcoclaurine
Catalog No.:BCN0458
CAS No.:1472-62-4
- 5-exo-Hydroxyborneol
Catalog No.:BCN0459
CAS No.:32751-73-8
- 3,8-Di-O-methylellagic acid 2-O-(5'-O-acetyl)arabinofuranoside
Catalog No.:BCN0460
CAS No.:365540-09-6
Xinnaokang improves cecal microbiota and lipid metabolism to target atherosclerosis.[Pubmed:34596907]
Lett Appl Microbiol. 2021 Oct 1.
This study aims to explore the potential mechanisms of Xinnaokang in atherosclerosis treatment. Firstly, the active components of Xinnaokang were analysed by HPLC, which contains ginsenoside Rg1, puerarin, tanshinone, notoginsenoside R1, ammonium glycyrrhizate and glycyrrhizin. Network pharmacology analysis showed there were 145 common targets of Xinnaokang, including the chemical stress, lipid metabolite, lipopolysaccharide, molecules of bacterial origin, nuclear receptor and fluid shear stress pathways. Then, the animal experiment showed that Xinnaokang reduced the body weight and blood lipid levels of atherosclerotic mice. Vascular plaque formation was increased in atherosclerotic mice, which was markedly reversed by Xinnaokang. In addition, Xinnaokang reduced CAV-1 expression and increased ABCA1, SREBP-1 and LXR expressions in the vasculature. Xinnaokang promoted SREBP-2 and LDLR expressions in the liver but decreased IDOL and PCSK9 expressions, indicating that Xinnaokang regulated lipid transport-related protein expression. Cecal microbiota diversity was reduced in atherosclerotic mice but increased after Xinnaokang treatment. Xinnaokang treatment also improved gut microbiota communities by enriching Actinobacteria, Bifidobacteriales and Bifidobacteriaceae abundances. Metabolic profile showed that Xinnaokang significantly reduced homogentisate, Phenylacetylglycine, alanine and methionine expressions in the liver of atherosclerotic mice. Xinnaokang effectively alleviated atherosclerosis, and this effect might be linked with the altered features of the liver metabolite profiles and cecal microbiota.
UPLC-MS/MS-Based Rat Serum Metabolomics Reveals the Detoxification Mechanism of Psoraleae Fructus during Salt Processing.[Pubmed:34567215]
Evid Based Complement Alternat Med. 2021 Sep 14;2021:5597233.
Psoraleae Fructus (PF) is a botanical medicine widely used in Asian countries, of which salt products have higher safety and efficacy. However, the biological mechanism of the detoxification of salt-processing Psoraleae Fructus (SPF) has not yet been revealed. In this study, UPLC-MS/MS technology was used to explore the metabolic differences between SPF and PF in normal rats and reveal the mechanism of salt processing. The histopathological results of rat liver and kidney showed that the degree of liver and kidney injure in the SPF group was less than that in the PF group. The results of metabolomics showed that the detoxification mechanism of PF by salt processing might be related to glycerophospholipid metabolism, phenylalanine, tyrosine, and tryptophan biosynthesis, arginine and proline metabolism, phenylalanine metabolism, and linoleic acid metabolism. PF-induced inflammation could be reduced by regulating the expression of metabolites to achieve the purpose of salt processing and detoxification. It included reducing the production of metabolites such as 1-acyl-sn-glycero-3-phosphocholine, sn-glycero-3-phosphocholine, tyrosine, arginine, linoleic acid, arachidonic acid, and Phenylacetylglycine/hippuric acid ratio and upregulating the expression of metabolites such as creatine.
Opisthorchis viverrini Infection Induces Metabolic and Fecal Microbial Disturbances in Association with Liver and Kidney Pathologies in Hamsters.[Pubmed:34270897]
J Proteome Res. 2021 Aug 6;20(8):3940-3951.
Opisthorchis viverrini (Ov) infection causes hepatobiliary diseases and is a major risk factor for cholangiocarcinoma. While several omics approaches have been employed to understand the pathogenesis of opisthorchiasis, effects of Ov infection on the host systemic metabolism and fecal microbiota have not been fully explored. Here, we used a (1)H NMR spectroscopy-based metabolic phenotyping approach to investigate Ov infection-induced metabolic disturbances at both the acute (1 month postinfection, 1 mpi) and chronic (4 mpi) stages in hamsters. A total of 22, 3, and 4 metabolites were found to be significantly different in the liver, serum, and urine, respectively, between Ov+ and Ov- groups. Elevated levels of hepatic amino acids and tricarboxylic acid (TCA)-cycle intermediates (fumarate and malate) were co-observed with liver injury in acute infection, whereas fibrosis-associated metabolites (e.g., glycine and glutamate) increased at the chronic infection stage. Lower levels of lipid signals ((CH2)n and CH2CH2CO) and higher levels of lysine and scyllo-inositol were observed in serum from Ov+ hamsters at 1 mpi compared to Ov- controls. Urinary levels of Phenylacetylglycine (a host-bacterial cometabolite) and tauro-beta-muricholic acid were higher in the Ov+ group, which coexisted with hepatic and mild kidney fibrosis. Furthermore, Ov+ animals showed higher relative abundances of fecal Methanobrevibacter (Archaea), Akkermansia, and Burkholderia-Paraburkholderia compared to the noninfected controls. In conclusion, along with liver and kidney pathologies, O. viverrini infection resulted in hepatic and mild renal pathologies, disturbed hepatic amino acid metabolism and the TCA cycle, and induced changes in the fecal microbial composition and urinary host-microbial cometabolism. This study provides the initial step toward an understanding of local and systemic metabolic responses of the host to O. viverrini infection.
Differential Metabolites in Chinese Autistic Children: A Multi-Center Study Based on Urinary (1)H-NMR Metabolomics Analysis.[Pubmed:34045978]
Front Psychiatry. 2021 May 11;12:624767.
Background: Autism spectrum disorder (ASD) is a group of early-onset neurodevelopmental disorders. However, there is no valuable biomarker for the early diagnosis of ASD. Our large-scale and multi-center study aims to identify metabolic variations between ASD and healthy children and to investigate differential metabolites and associated pathogenic mechanisms. Methods: One hundred and seventeen autistic children and 119 healthy children were recruited from research centers of 7 cities. Urine samples were assayed by (1)H-NMR metabolomics analysis to detect metabolic variations. Multivariate statistical analysis, including principal component analysis (PCA), and orthogonal projection to latent structure discriminant analysis (OPLS-DA), as well as univariate analysis were used to assess differential metabolites between the ASD and control groups. The differential metabolites were further analyzed by receiver operating characteristics (ROC) curve analysis and metabolic pathways analysis. Results: Compared with the control group, the ASD group showed higher levels of glycine, guanidinoacetic acid, creatine, hydroxyPhenylacetylglycine, Phenylacetylglycine, and formate and lower levels of 3-aminoisobutanoic acid, alanine, taurine, creatinine, hypoxanthine, and N-methylnicotinamide. ROC curve showed relatively significant diagnostic values for hypoxanthine [area under the curve (AUC) = 0.657, 95% CI 0.588 to 0.726], creatinine (AUC = 0.639, 95% CI 0.569 to 0.709), creatine (AUC = 0.623, 95% CI 0.552 to 0.694), N-methylnicotinamide (AUC = 0.595, 95% CI 0.523 to 0.668), and guanidinoacetic acid (AUC = 0.574, 95% CI 0.501 to 0.647) in the ASD group. Combining the metabolites creatine, creatinine and hypoxanthine, the AUC of the ROC curve reached 0.720 (95% CI 0.659 to 0.777). Significantly altered metabolite pathways associated with differential metabolites were glycine, serine and threonine metabolism, arginine and proline metabolism, and taurine and hypotaurine metabolism. Conclusions: Urinary amino acid metabolites were significantly altered in children with ASD. Amino acid metabolic pathways might play important roles in the pathogenic mechanisms of ASD.
Roux-en-Y gastric bypass surgery in Zucker rats induces bacterial and systemic metabolic changes independent of caloric restriction-induced weight loss.[Pubmed:33535876]
Gut Microbes. 2021 Jan-Dec;13(1):1-20.
Mechanisms of Roux-en-Y gastric bypass (RYGB) surgery are not fully understood. This study aimed to investigate weight loss-independent bacterial and metabolic changes, as well as the absorption of bacterial metabolites and bile acids through the hepatic portal system following RYGB surgery. Three groups of obese Zucker (fa/fa) rats were included: RYGB (n = 11), sham surgery and body weight matched with RYGB (Sham-BWM, n = 5), and sham surgery fed ad libitum (Sham-obese, n = 5). Urine and feces were collected at multiple time points, with portal vein and peripheral blood obtained at the end of the study. Metabolic phenotyping approaches and 16S rRNA gene sequencing were used to determine the biochemical and bacterial composition of the samples, respectively. RYGB surgery-induced distinct metabolic and bacterial disturbances, which were independent of weight loss through caloric restriction. RYGB resulted in lower absorption of phenylalanine and choline, and higher urinary concentrations of host-bacterial co-metabolites (e.g., Phenylacetylglycine, indoxyl sulfate), together with higher fecal trimethylamine, suggesting enhanced bacterial aromatic amino acid and choline metabolism. Short chain fatty acids (SCFAs) were lower in feces and portal vein blood from RYGB group compared to Sham-BWM, accompanied with lower abundances of Lactobacillaceae, and Ruminococcaceae known to contain SCFA producers, indicating reduced bacterial fiber fermentation. Fecal gamma-amino butyric acid (GABA) was found in higher concentrations in RYGB than that in Sham groups and could play a role in the metabolic benefits associated with RYGB surgery. While no significant difference in urinary BA excretion, RYGB lowered both portal vein and circulating BA compared to Sham groups. These findings provide a valuable resource for how dynamic, multi-systems changes impact on overall metabolic health, and may provide potential therapeutic targets for developing downstream non-surgical treatment for metabolic disease.
Novel Metabolic Signatures of Prostate Cancer Revealed by (1)H-NMR Metabolomics of Urine.[Pubmed:33498542]
Diagnostics (Basel). 2021 Jan 20;11(2). pii: diagnostics11020149.
Prostate cancer (PC) is one of the most common male cancers worldwide. Until now, there is no consensus about using urinary metabolomic profiling as novel biomarkers to identify PC. In this study, urine samples from 50 PC patients and 50 non-cancerous individuals (control group) were collected. Based on (1)H nuclear magnetic resonance ((1)H-NMR) analysis, 20 metabolites were identified. Subsequently, principal component analysis (PCA), partial least squares-differential analysis (PLS-DA) and ortho-PLS-DA (OPLS-DA) were applied to find metabolites to distinguish PC from the control group. Furthermore, Wilcoxon test was used to find significant differences between the two groups in metabolite urine levels. Guanidinoacetate, Phenylacetylglycine, and glycine were significantly increased in PC, while L-lactate and L-alanine were significantly decreased. The receiver operating characteristics (ROC) analysis revealed that the combination of guanidinoacetate, Phenylacetylglycine, and glycine was able to accurately differentiate 77% of the PC patients with sensitivity = 80% and a specificity = 64%. In addition, those three metabolites showed significant differences in patients stratified for Gleason score 6 and Gleason score >/=7, indicating potential use to detect significant prostate cancer. Pathway enrichment analysis using the KEGG (Kyoto Encyclopedia of Genes and Genomes) and the SMPDB (The Small Molecule Pathway Database) revealed potential involvement of KEGG "Glycine, Serine, and Threonine metabolism" in PC. The present study highlights that guanidinoacetate, Phenylacetylglycine, and glycine are potential candidate biomarkers of PC. To the best knowledge of the authors, this is the first study identifying guanidinoacetate, and Phenylacetylglycine as potential novel biomarkers in PC.
The gut microbial metabolite phenylacetylglycine protects against cardiac injury caused by ischemia/reperfusion through activating beta2AR.[Pubmed:33307065]
Arch Biochem Biophys. 2021 Jan 15;697:108720.
BACKGROUND: Myocardial ischemia/reperfusion (I/R) injury is closely related to cardiomyocyte apoptosis. Stimulating beta2 adrenergic receptor (beta2AR) can effectively combat cardiomyocyte apoptosis. Previous studies demonstrate that the gut microbial metabolite Phenylacetylglycine (PAGly) can stimulate beta2AR. However, the effect of PAGly on myocardial I/R injury remains unknown. METHODS: The hypoxia/reoxygenation (H/R) model was established using the neonatal mouse cardiomyocytes (NMCMs). Different doses of PAGly were used to treat NMCMs, and apoptosis was detected by terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) staining. Additionally, the level of cyclic adenosine monophosphate (cAMP) was examined by using a cAMP detection kit. Mouse model of myocardial I/R injury was established in C57BL/6 mice, and different doses of phenylacetic acid were administrated intraperitoneally. Apoptosis of myocardial cells was detected by TUNEL and alpha-actin staining. The area at risk and the infarct areas were identified by 2,3,5-triphenyltetrazolium chloride (TTC) and Evans blue staining. Western blotting was used to measure the protein expression levels of phosphorylated phosphatidylinositol 3-kinase (p-PI3K), total Akt (t-Akt), phosphorylated Akt (p-AKT), Bcl-2-associated X protein (Bax), B-cell lymphoma-2 (Bcl-2), cleaved caspase-3. RESULTS: PAGly significantly suppressed H/R injury-induced apoptosis in NMCMs and inhibited apoptosis in myocardial I/R injured mice in vivo. We verified that PAGly activated the anti-apoptotic Galphai/PI3K/AKT signaling cascade in NMCMs via stimulating beta2AR signaling. Continuous administration of PAGly at an appropriate dose could inhibit apoptosis and reduce the infarct size resulting from I/R injury in mice. However, high-dose PAGly treatment was associated with a higher mortality rate. Moreover, we demonstrated that Aspirin reduced the infarct size and the high mortality caused by high doses of PAGly in I/R injured mice. CONCLUSIONS: These findings suggest that treatment with the gut microbial metabolite PAGly could suppress cardiomyocyte apoptosis caused by myocardial I/R injury and reduce the infarct size, which provides a novel therapeutic strategy for patients with myocardial infarction.
Metabolomics of Interstitial Fluid, Plasma and Urine in Patients with Arterial Hypertension: New Insights into the Underlying Mechanisms.[Pubmed:33187152]
Diagnostics (Basel). 2020 Nov 11;10(11). pii: diagnostics10110936.
There is growing evidence that lymphatic system plays a pivotal role in the pathogenesis of hypertension. Here, for the first time, the metabolome of interstitial fluid is analyzed in patients with arterial hypertension. Due to ethical issues to obtain human interstitial fluid samples, this study included only oncological patients after axillary lymph node dissection (ALND). These patients were matched into hypertensive (n = 29) and normotensive (n = 35) groups with similar oncological status. Simultaneous evaluation of interstitial fluid, plasma, and urine was obtained by combining high-resolution proton nuclear magnetic resonance ((1)H NMR) spectroscopy with chemometric analysis. Orthogonal partial least squares discriminant analysis (OPLS-DA) provided a clear differentiation between the hypertension and normotensive group, with the discrimination visible in each biofluid. In interstitial fluid nine potential metabolomic biomarkers for hypertension could be identified (creatinine, proline, pyroglutamine, glycine, alanine, 1-methylhistidine, the lysyl group of albumin, threonine, lipids), seven distinct markers in plasma (creatinine, mannose, isobutyrate, glycine, alanine, lactate, acetate, ornithine), and seven respectively in urine (methylmalonate, citrulline, Phenylacetylglycine, fumarate, citrate, 1-methylnicotinamide, trans-aconitate). Biomarkers in plasma and urine allowed for the identification of specific biochemical pathways involved in hypertension, as previously suggested. Analysis of the interstitial fluid metabolome provided additional biomarkers compared to plasma or urine. Those biomarkers reflected primarily alterations in the metabolism of lipids and amino acids, and indicated increased levels of oxidative stress/inflammation in patients with hypertension.
Potential Biomarkers for Early Detection of 3-MCPD Dipalmitate Exposure in Sprague-Dawley Rats.[Pubmed:32786827]
J Agric Food Chem. 2020 Sep 2;68(35):9594-9602.
3-Chloro-1,2-propandiol (3-MCPD) dipalmitate is one of the major 3-MCPD esters formed during food processing. In this single-dose study, the metabonomic profile changes in the 48 h after orally administrated 3-MCPD dipalmitate at 1600 mg/kg BW to Sprague-Dawley (SD) rats were determined with liquid chromatography (LC) coupled with mass spectrometry (MS) system. The chemical structures of 12 potential biomarkers for 3-MCPD dipalmitate exposures early detection were detected and tentatively identified from the plasma of SD rats, including indoxyl sulfate, phenol sulfate, p-cresol sulfate, 2-phenylethanol glucuronide, p-cresol glucuronide, p-cresol, allantoin, Phenylacetylglycine, pyrocatechol sulfate, phenyllactic acid, 5-hydroxyindoleacetic acid, and creatinine. Taking into account the metabolites identified from SD rats' kidney, liver, testes, and spleen samples, 3-MCPD dipalmitate might potentially disturb the phenylalanine, tryptophan, tyrosine, glycine, fatty acid, and purine metabolisms. The results suggested that the 12 plasma metabolites could be potentially applied in detecting the early exposures of 3-MCPD esters.
The protective effect of Xanthoceras sorbifolia Bunge husks on cognitive disorder based on metabolomics and gut microbiota analysis.[Pubmed:32634462]
J Ethnopharmacol. 2021 Oct 28;279:113094.
AIM OF THE STUDY: The husks of Xanthoceras sorbifolia Bunge mainly used in north China as folk medicine were reported to have potential protective effect on cognitive impairment. However, the mechanism remains unclear. In order to fully understand the mechanism of the protection, a complementary study of the husks was conducted. MATERIALS AND METHODS: The urinary and fecal metabolomics were used to analyze the potential biomarkers by the liquid chromatography-tandem time of flight mass spectrometry, and the16S rDNA technology was applied to conduct the analysis of microbiota species in the fecal samples of the rats, which is a significant influencing factor for the development of cognitive impairment. RESULTS: In metabolomics study, ten potential metabolic biomarkers, which are hippuric acid, kynurenic acid, creatinine, phenylalanine, xanthurenic acid, Phenylacetylglycine, succinyladenosine, cresol sulfate, tryptophan 2-C-mannoside and N4-Acetylcytidine in urine, along with two, including isoleucine and phenylalanine in feces, were preliminarily identified, involving multiple pathways such as tryptophan, purine, kynurenine, and phenylalanine metabolism. The perturbation of these metabolic pathways could be related with insulin resistance, oxidative stress, energy metabolism deficit and neuroinflammation, which were risk factors to cause cognitive impairment. In gut microbiota analysis, the relative abundance of c_Bacteroidia, c_Alphaproteobacteria, f_Prevotellaceae, f_Sphingomonadaceae, f_Burkholderiaceae, g_Prevotellaceae_NK3B31_group and p_Bacteroidetes was significantly changed in the rats with cognitive impairment. Spearman's analysis showed obvious correlation between the metabolites and the microbiota species. In the rats with pretreatment of the husks extract, metabolites maintained a relative normal level, and the husks extract could regulate the gut microbiota, especially f_Prevotellaceae and g_Prevotellaceae_NK3B31_group, indicating the effect of the husks on the metabolic pathways via GMs. Such amino acids as isoleucine and phenylalanine failed to show any significant correlation with the microbiota species, indicating that the husks exhibited the potential protective effect through gut microbiota and other pathways. CONCLUSIONS: The husks extract could improve the intestinal microenvironment, and the stability of intestinal microenvironment was associated with normality of tryptophan, purine, kynurenine and phenylalanine metabolic pathways etc, which probably had an effect on cognitive function. This complementary work suggested that gut microbiotas were potential targets of the husks to exert its effect on cognitive impairment.
Dual Metabolomic Platforms Identified a Novel Urinary Metabolite Signature for Hepatitis B Virus-Infected Patients with Depression.[Pubmed:32547129]
Diabetes Metab Syndr Obes. 2020 May 18;13:1677-1683.
Objective: Depression could make the treatment outcome worse. However, up to now, no objective methods were developed to diagnose depression in hepatitis B virus (HBV)-infected patients. Therefore, the dual metabolomic platforms were used here to identify potential biomarkers for diagnosing HBV-infected patients with depression (dHB). Methods: Both gas chromatography-mass spectrometry-based and nuclear magnetic resonance-based metabolomic platforms were used to conduct urine metabolic profiling of dHB subjects and HBV-infected patients without depression (HB). Orthogonal partial least-squares discriminant analysis was used to identify the differential metabolites between dHB subjects and HB subjects, and the step-wise logistic regression analysis was used to identify potential biomarkers. Results: In total, 21 important metabolites responsible for distinguishing dHB subjects from HB subjects were identified. Meanwhile, seven potential biomarkers (alpha-ydroxyisobutyric acid, hippuric acid, azelaic acid, isobutyric acid, malonic acid, levulinic acid, and Phenylacetylglycine) were viewed as potential biomarkers. The simplified biomarker panel consisting of these seven metabolites had an excellent diagnostic performance in discriminating dHB subjects from HB subjects. Moreover, this panel could yield a higher accuracy in separating dHB subjects from HB subjects than our previous panels (identified by single metabolomic platform) did. Conclusion: These results suggested that the dual metabolomic platforms could yield a better urinary biomarker panel for dHB subjects than any single metabolomic platform did, and our results could be helpful for developing an objective method in future to diagnose depression in HBV-infected patients.
Net release and uptake of xenometabolites across intestinal, hepatic, muscle, and renal tissue beds in healthy conscious pigs.[Pubmed:32538141]
Am J Physiol Gastrointest Liver Physiol. 2020 Aug 1;319(2):G133-G141.
Xenometabolites from microbial and plant sources are thought to confer beneficial as well as deleterious effects on host physiology. Studies determining absorption and tissue uptake of xenometabolites are limited. We utilized a conscious catheterized pig model to evaluate interorgan flux of annotated known and suspected xenometabolites, derivatives, and bile acids. Female pigs (n = 12, 2-3 mo old, 25.6 +/- 2.2 kg) had surgically implanted catheters across portal-drained viscera (PDV), splanchnic compartment (SPL), liver, kidney, and hindquarter muscle. Overnight-fasted arterial and venous plasma was collected simultaneously in a conscious state and stored at -80 degrees C. Thawed samples were analyzed by liquid chromatography-mass spectrometry. Plasma flow was determined with para-aminohippuric acid dilution technology and used to calculate net organ balance for each metabolite. Significant organ uptake or release was determined if net balance differed from zero. A total of 48 metabolites were identified in plasma, and 31 of these had at least one tissue with a significant net release or uptake. All bile acids, indole-3-acetic acid, indole-3-arylic acid, and hydrocinnamic acid were released from the intestine and taken up by the liver. Indole-3-carboxaldehyde, p-cresol glucuronide, 4-hydroxyphenyllactic acid, dodecanendioic acid, and Phenylacetylglycine were also released from the intestines. Liver or kidney uptake was noted for indole-3-acetylglycine, p-cresol glucuronide, atrolactic acid, and dodecanedioic acid. Indole-3-carboxaldehyde, atrolactic acid, and dodecanedioic acids showed net release from skeletal muscle. The results confirm gastrointestinal origins for several known xenometabolites in an in vivo overnight-fasted conscious pig model, whereas nongut net release of other putative xenometabolites suggests a more complex metabolism.NEW & NOTEWORTHY Xenometabolites from microbe origins influence host health and disease, but absorption and tissue uptake of these metabolites remain speculative. Results herein are the first to demonstrate in vivo organ uptake and release of these metabolites. We used a conscious catheterized pig model to confirm gastrointestinal origins for several xenometabolites (e.g., indolic compounds, 4-hydroxyphenyllactic acid, dodecanendioic acid, and phenylacetylgycine). Liver and kidney were major sites for xenometabolite uptake, likely highlighting liver conjugation metabolism and renal excretion.
Investigating the role of endogenous opioid system in chloroquine-induced phospholipidosis in rat liver by morphological, biochemical and molecular modelling studies.[Pubmed:32367550]
Clin Exp Pharmacol Physiol. 2020 Sep;47(9):1575-1583.
Drug-induced phospholipidosis (DIPL) is characterized by phospholipid storage in the lysosomes of affected tissues. Many severe effects and toxicities have been linked to DIPL. The aim of this study was to determine whether the endogenous opioid system is involved in chloroquine-induced phospholipidosis. The effect of naltrexone as an antagonist of opioid receptors in chloroquine-induced phospholipidosis in rat liver was investigated by morphological, biochemical, and molecular modelling studies. Transmission electron microscopy (TEM) showed that morphological characteristic changes of rat liver, including the number of lamellar bodies, grade of vacuolization and cell steatosis, were markedly attenuated in rats treated with naltrexone alone or in combination with chloroquine, in comparison with chloroquine-treated rats. The results of liquid chromatography mass spectrometry (LC/MS) showed that the concentrations of Phenylacetylglycine (PAG) and hippuric acid (HA) were significantly decreased and increased, respectively, in target groups. Besides, the concentration ratio of PAG/HA was significantly decreased. Spectrophotometry resulted in a notable decrease in alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities in target groups. The results from the molecular docking and molecular dynamic simulation studies demonstrated clear chloroquine interaction with the active site cavity of the micro opioid receptor. These data suggest that administration of naltrexone alone, or in combination with chloroquine, notably attenuates the side effects of chloroquine-induced phospholipidosis, as well as demonstrating an increased probability of the endogenous opioid system involvement in chloroquine-induced phospholipidosis in rat liver.
Developmental Signatures of Microbiota-Derived Metabolites in the Mouse Brain.[Pubmed:32344839]
Metabolites. 2020 Apr 25;10(5). pii: metabo10050172.
The gut microbiome is recognized to exert a wide-ranging influence on host health and disease, including brain development and behavior. Commensal bacteria can produce bioactive molecules that enter the circulation and impact host physiology and homeostasis. However, little is known about the potential for these metabolites to cross the blood-brain barrier and enter the developing brain under normal physiological conditions. In this study, we used a liquid chromatography-mass spectrometry-based metabolomic approach to characterize the developmental profiles of microbial-derived metabolites in the forebrains of mice across three key postnatal developmental stages, co-occurring with the maturation of the gut microbiota. We demonstrate that direct metabolites of the gut microbiome (e.g., imidazole propionate) or products of the combinatorial metabolism between the microbiome and host (e.g., 3-indoxyl-sulfate, trimethylamine-N-oxide, and Phenylacetylglycine) are present in the forebrains of mice as early as the neonatal period and remain into adulthood. These findings demonstrate that microbial-associated molecules can cross the BBB either in their detected form or as precursor molecules that undergo further processing in the brain. These chemical messengers are able to bind receptors known to be expressed in the brain. Alterations in the gut microbiome may therefore influence neurodevelopmental trajectories via the regulation of these microbial-associated metabolites.
Metabolomic Analysis Reveals Distinct Profiles in the Plasma and Urine Associated with IgE Reactions in Childhood Asthma.[Pubmed:32213896]
J Clin Med. 2020 Mar 24;9(3). pii: jcm9030887.
Several metabolomics studies have identified altered metabolic pathways that are related to asthma. However, an integrative analysis of the metabolic responses across blood and urine for a comprehensive framework of asthma in early childhood remains lacking. Fifty-four age-matched children with asthma (n = 28) and healthy controls (n = 26) were enrolled. Metabolome analysis of the plasma and urine samples was performed using (1)H-nuclear magnetic resonance (NMR) spectroscopy coupled with partial least-squares discriminant analysis (PLS-DA). Integrated analysis of blood and urine metabolic profiling related to IgE reactions for childhood asthma was investigated. A significantly higher plasma histidine level was found, in parallel with lower urinary 1-methylnicotinamide and trimethylamine N-oxide (TMAO) levels, in children with asthma compared to healthy controls. Compared to children without allergic sensitization, 11 (92%) plasma metabolites and 8 (80%) urinary metabolites were found to be significantly different in children with IgE and food sensitization respectively. There were significant correlations between the plasma 3-hydroxybutyric acid and excreted volumes of the hydroxy acids, which were strongly correlated to plasma leucine and valine levels. Urine N-Phenylacetylglycine, a microbial-host co-metabolite, was strongly correlated with total serum and food allergen-specific IgE levels. Plasma pyruvate and urine valine, leucine, and isoleucine degradation metabolisms were significantly associated with allergic sensitization for childhood asthma. In conclusion, blood and urine metabolome reflect different metabolic pathways in allergic reactions. Plasma pyruvate metabolism to acetic acid appears to be associated with serum IgE production, whereas urine branched-chain amino acid metabolism primarily reflects food allergic reactions against allergies.


