Boc- D-1-Nal-OHCAS# 55447-00-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55447-00-2 | SDF | Download SDF |
| PubChem ID | 7021841 | Appearance | Powder |
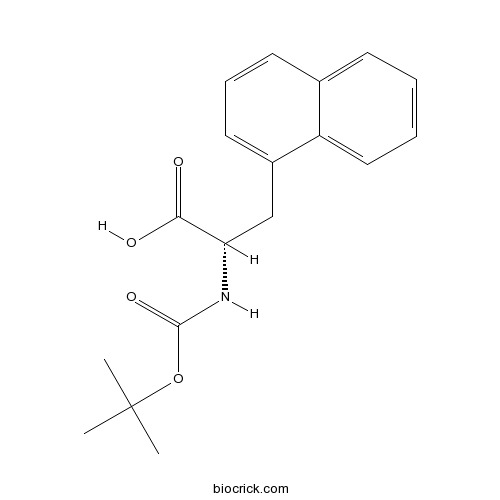
| Formula | C18H21NO4 | M.Wt | 315.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-naphthalen-1-ylpropanoic acid | ||
| SMILES | CC(C)(C)OC(=O)NC(CC1=CC=CC2=CC=CC=C21)C(=O)O | ||
| Standard InChIKey | KHHIGWRTNILXLL-HNNXBMFYSA-N | ||
| Standard InChI | InChI=1S/C18H21NO4/c1-18(2,3)23-17(22)19-15(16(20)21)11-13-9-6-8-12-7-4-5-10-14(12)13/h4-10,15H,11H2,1-3H3,(H,19,22)(H,20,21)/t15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc- D-1-Nal-OH Dilution Calculator

Boc- D-1-Nal-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1706 mL | 15.8529 mL | 31.7058 mL | 63.4115 mL | 79.2644 mL |
| 5 mM | 0.6341 mL | 3.1706 mL | 6.3412 mL | 12.6823 mL | 15.8529 mL |
| 10 mM | 0.3171 mL | 1.5853 mL | 3.1706 mL | 6.3412 mL | 7.9264 mL |
| 50 mM | 0.0634 mL | 0.3171 mL | 0.6341 mL | 1.2682 mL | 1.5853 mL |
| 100 mM | 0.0317 mL | 0.1585 mL | 0.3171 mL | 0.6341 mL | 0.7926 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc- D-1-Nal-OH
- 8beta-(4-Hydroxytigloyloxy)ovatifolin
Catalog No.:BCN7122
CAS No.:554449-27-3
- Deferasirox Fe3+ chelate
Catalog No.:BCC1521
CAS No.:554435-83-5
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- Lithium carbonate
Catalog No.:BCC7970
CAS No.:554-13-2
- Hydroxyisoleucine
Catalog No.:BCN8402
CAS No.:55399-93-4
- Baohuoside II
Catalog No.:BCN2888
CAS No.:55395-07-8
- Vidarabine
Catalog No.:BCC4877
CAS No.:5536-17-4
- Beclomethasone dipropionate
Catalog No.:BCC4257
CAS No.:5534-09-8
- Petasiphenone
Catalog No.:BCC8100
CAS No.:162616-81-1
- Soyasaponin II
Catalog No.:BCN1418
CAS No.:55319-36-3
- Atherosperminine
Catalog No.:BCN8208
CAS No.:5531-98-6
- Costunolide
Catalog No.:BCN5740
CAS No.:553-21-9
- Anisodamine hydrobromide
Catalog No.:BCC8119
CAS No.:55449-49-5
- Z-Asp(OtBu)-OH.H2O
Catalog No.:BCC2789
CAS No.:5545-52-8
- Chimonanthine
Catalog No.:BCN7824
CAS No.:5545-89-1
- Jujuboside A
Catalog No.:BCN4949
CAS No.:55466-04-1
- Jujuboside B
Catalog No.:BCN4950
CAS No.:55466-05-2
- Indole-3-glyoxylamide
Catalog No.:BCN6802
CAS No.:5548-10-7
- Isosaxalin
Catalog No.:BCN5741
CAS No.:55481-86-2
- Mollugin
Catalog No.:BCN5742
CAS No.:55481-88-4
- Nepetoidin B
Catalog No.:BCN7082
CAS No.:55486-06-1
- Myriceric acid B
Catalog No.:BCN5743
CAS No.:55497-79-5
- Methyldopa
Catalog No.:BCC4676
CAS No.:555-30-6
- Tritetradecanoin
Catalog No.:BCN8389
CAS No.:555-45-3
Efficient synthesis of (S)-N-Boc-3-hydroxypiperidine using an (R)-specific carbonyl reductase from Candida parapsilosis.[Pubmed:28243985]
World J Microbiol Biotechnol. 2017 Mar;33(3):61.
(S)-N-Boc-3-hydroxypiperidine (S-NBHP) is a critical chiral intermediate in the synthesis of pharmaceuticals, including ibrutinib, the active pharmaceutical ingredient of the new drug Imbruvica approved for the treatment of lymphoma. An (R)-specific carbonyl reductase from Candida parapsilosis (CprCR, also known as R-specific alcohol dehydrogenase) that catalyzes asymmetric reduction to produce (S)-N-Boc-3-hydroxypiperidine (S-NBHP) was identified for the first time. When co-expressed with a glucose dehydrogenase from Bacillus megaterium in Escherichia coli Rosetta (DE3), recombinant crude enzyme exhibited an activity of 9 U/mg with N-Boc-3-piperidone as the substrate and 12 U/mg with glucose as the substrate. The biocatalysis of N-Boc-3-piperidone to S-NBHP using recombinant whole-cell biocatalysts was processed in a water/butyl acetate system as well as an aqueous monophasic system without extra NAD(+)/NADH. This process showed great commercial potential, with a 100 g/l substrate concentration and a whole cells loading (w/v) of 10%, with the conversion of 97.8% and an e.e. of 99.8% in an aqueous monophasic system.
Tuning the Molar Composition of "Charge-Shifting" Cationic Copolymers Based on 2-(N,N-Dimethylamino)Ethyl Acrylate and 2-(tert-Boc-Amino)Ethyl Acrylate.[Pubmed:28045212]
Macromol Rapid Commun. 2017 Mar;38(5).
Copolymers of 2-(N,N-dimethylamino)ethyl acrylate (DMAEA) and 2-(tert-Boc-amino)ethyl acrylate (tBocAEA) are synthesized by reversible addition-fragmentation chain transfer polymerization in a controlled manner with defined molar masses and narrow molar masses distributions (Eth
Rhodium(III)-Catalyzed Ortho-Alkenylation of Anilines Directed by a Removable Boc-Protecting Group.[Pubmed:28322570]
Org Lett. 2017 Apr 7;19(7):1800-1803.
The rhodium(III)-catalyzed ortho-alkenylation of N-Boc-anilines with alkenes such as acrylate ester and styrene proceeds smoothly through C-H bond cleavage. Obtained o-alkenylanilines can be readily transformed to nitrogen-containing fused heteroaromatic compounds including indoles and quinolines.
Effects on Energy Metabolism of Two Guanidine Molecules, (Boc)2 -Creatine and Metformin.[Pubmed:28128472]
J Cell Biochem. 2017 Sep;118(9):2700-2711.
Several enzymes are involved in the energy production, becoming a possible target for new anti-cancer drugs. In this paper, we used biochemical and in silico studies to evaluate the effects of two guanidine molecules, (Boc)2 -creatine and metformin, on creatine kinase, an enzyme involved in the regulation of intracellular energy levels. Our results show that both drugs inhibit creatine kinase activity; however, (Boc)2 -creatine displays a competitive inhibition, while metformin acts with a non-competitive mechanism. Moreover, (Boc)2 -creatine is able to inhibit the activity of hexokinase with a non-competitive mechanism. Considering that creatine kinase and hexokinase are involved in energy metabolism, we evaluated the effects of (Boc)2 -creatine and metformin on the ATP/AMP ratio and on cellular proliferation in healthy fibroblasts, human breast cancer cells (MDA-MB-468), a human neuroblastoma cell line (SH-SY5Y), a human Hodgkin lymphoma cell line (KMH2). We found that healthy fibroblasts were only partially affected by (Boc)2 -creatine, while both ATP/AMP ratio and viability of the three cancer cell lines were significantly decreased. By inhibiting both creatine kinase and hexokinase, (Boc)2 -creatine appears as a promising new agent in anticancer treatment. Further research is needed to understand what types of cancer cells are most suitable to treatment by this new compound. J. Cell. Biochem. 118: 2700-2711, 2017. (c) 2017 Wiley Periodicals, Inc.


