Bisisorhapontigenin ECAS# N/A |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | N/A | SDF | Download SDF |
| PubChem ID | 11497197 | Appearance | Powder |
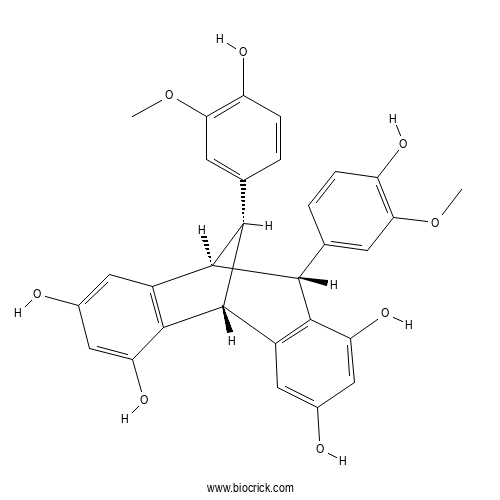
| Formula | C30H26O8 | M.Wt | 514.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,8R,9S,16S)-8,16-bis(4-hydroxy-3-methoxyphenyl)tetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene-4,6,12,14-tetrol | ||
| SMILES | COC1=C(C=CC(=C1)C2C3C(C4=C(C2C5=C3C=C(C=C5O)O)C=C(C=C4O)O)C6=CC(=C(C=C6)O)OC)O | ||
| Standard InChIKey | JJWYSQXEIGBPJQ-ZIMFAVFZSA-N | ||
| Standard InChI | InChI=1S/C30H26O8/c1-37-23-7-13(3-5-19(23)33)25-27-17(9-15(31)11-21(27)35)30-26(14-4-6-20(34)24(8-14)38-2)29(25)18-10-16(32)12-22(36)28(18)30/h3-12,25-26,29-36H,1-2H3/t25-,26+,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bisisorhapontigenin E Dilution Calculator

Bisisorhapontigenin E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9436 mL | 9.7182 mL | 19.4363 mL | 38.8727 mL | 48.5909 mL |
| 5 mM | 0.3887 mL | 1.9436 mL | 3.8873 mL | 7.7745 mL | 9.7182 mL |
| 10 mM | 0.1944 mL | 0.9718 mL | 1.9436 mL | 3.8873 mL | 4.8591 mL |
| 50 mM | 0.0389 mL | 0.1944 mL | 0.3887 mL | 0.7775 mL | 0.9718 mL |
| 100 mM | 0.0194 mL | 0.0972 mL | 0.1944 mL | 0.3887 mL | 0.4859 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Delphinidin 3-O-galactoside
Catalog No.:BCN9089
CAS No.:197250-28-5
- (-)-Isobicyclogermacrenal
Catalog No.:BCN9088
CAS No.:73256-82-3
- Cyclohexanecarboxylic acid, 3-[[(2E)-3-[4-(D-glucopyranosyloxy)-3-hydroxyphenyl]-1-oxo-2-propen-1-yl...
Catalog No.:BCN9087
CAS No.:1629852-63-6
- Cucurbitacin Q1
Catalog No.:BCN9086
CAS No.:99530-82-2
- (3r)-7,2'-Dihydroxy-3',4'-dimethoxyisoflavan
Catalog No.:BCN9085
CAS No.:64474-51-7
- (-)-Sesamin
Catalog No.:BCN9084
CAS No.:13079-95-3
- (3β,7β,12β,20Z )- 3,7,12- trihydroxy-11,15,23-trioxo-lanost-8,20-dien-26-oic acid
Catalog No.:BCN9083
CAS No.:1961358-02-0
- Calenduloside E
Catalog No.:BCN9082
CAS No.:26020-14-4
- Chol-8-en-24-oic acid, 7,15-dihydroxy-4,4,14-trimethyl-3,11-dioxo-, (5α)-
Catalog No.:BCN9081
CAS No.:942936-54-1
- Lanosta-8,20(22)-dien-26-oic acid, 15-hydroxy-3,11,23-trioxo-, (15α,20Z)-
Catalog No.:BCN9080
CAS No.:1961358-01-9
- Sodium deoxycholate
Catalog No.:BCN9079
CAS No.:302-95-4
- Sodium taurocholate
Catalog No.:BCN9078
CAS No.:145-42-6
- Bisabolone oxide A
Catalog No.:BCN9091
CAS No.:22567-38-0
- (5S,6S,7S,8R)-8-Chloro-5,6,7-trihydroxy-2-phenylethyl-5,6,7,8-tetrahydro-4H-chromen-4-one
Catalog No.:BCN9092
CAS No.:626236-06-4
- Ephedrine
Catalog No.:BCN9093
CAS No.:299-42-3
- Cyanidin 3-O- galactopyranoside
Catalog No.:BCN9094
CAS No.:142506-26-1
- Febrifugine dihydrochloride
Catalog No.:BCN9095
CAS No.:32434-42-7
- Malvidin 3-O-glucoside
Catalog No.:BCN9096
CAS No.:18470-06-9
- Delphinidin 3-O-glucoside
Catalog No.:BCN9097
CAS No.:50986-17-9
- Petunidin 3-O-glucoside
Catalog No.:BCN9098
CAS No.:71991-88-3
- α-Terpinene
Catalog No.:BCN9099
CAS No.:99-86-5
- Cyanidin 3-O-arabinoside
Catalog No.:BCN9100
CAS No.:792868-19-0
- Bisisorhapontigenin F
Catalog No.:BCN9101
CAS No.:
- 3-(2-Hydroxy-4,6-dimethoxyphenyl)-1-(4-hydroxyphenyl)-1-propanone
Catalog No.:BCN9102
CAS No.:151752-07-7
Functional expression of a novel methanol-stable esterase from Geobacillus subterraneus DSM13552 for biocatalytic synthesis of cinnamyl acetate in a solvent-free system.[Pubmed:32600313]
BMC Biotechnol. 2020 Jun 29;20(1):36.
BACKGROUND: Esterases are widely distributed in nature and have important applications in medical, industrial and physiological. Recently, the increased demand for flavor esters has prompted the search of catalysts like lipases and esterases. Esterases from thermophiles also show thermal stability at elevated temperatures and have become enzymes of special interest in biotechnological applications. Although most of esterases catalyzed reactions are carried out in toxic and inflammable organic solvents, the solvent-free system owning many advantages such as low cost and easy downstream processing. RESULTS: The gene estGSU753 from Geobacillus subterraneus DSM13552 was cloned, sequenced and overexpressed into Escherichia coli BL21 (DE3). The novel gene has an open reading frame of 753 bp and encodes 250-amino-acid esterase (EstGSU753). The sequence analysis showed that the protein contains a catalytic triad formed by Ser97, Asp196 and His226, and the Ser of the active site is located in the conserved motif Gly95-X-Ser97-X-Gly99 included in most esterases and lipases. The protein catalyzed the hydrolysis of pNP-esters of different acyl chain lengths, and the enzyme specific activity was 70 U/mg with the optimum substrate pNP-caprylate. The optimum pH and temperature of the recombinant enzyme were 8.0 and 60 degrees C respectively. The resulting EstGSU753 showed remarkable stability against methanol. After the incubation at 50% methanol for 9 days, EstGSU753 retained 50% of its original activity. Even incubation at 90% methanol for 35 h, EstGSU753 retained 50% of its original activity. Also, the preliminary study of the transesterification shows the potential value in synthesis of short-chain flavor esters in a solvent-free system, and more than 99% conversion was obtained in 6 h (substrate: cinnamyl alcohol, 1.0 M). CONCLUSIONS: This is the first report of esterase gene cloning from Geobacillus subterraneus with detailed enzymatic properties. This methanol-stable esterase showed potential value in industrial applications especially in the perfume industry.
In vitro antimicrobial combinatory effect of Cinnamomum cassia essential oil with 8-hydroxyquinoline against Staphylococcus aureus in liquid and vapour phase.[Pubmed:32350955]
J Appl Microbiol. 2020 Apr 29.
AIMS: The objective of the study was to evaluate the antimicrobial interactions between two volatile agents, Cinnamomum cassia essential oil (CCEO) and 8-hydroxyquinoline (8-HQ) against Staphylococcus aureus strains in liquid and vapour phases. METHODS AND RESULTS: In vitro antimicrobial effect of CCEO in combination with 8-HQ was evaluated against 12 strains of S. aureus by broth volatilization chequerboard method. Results show additive effects against all S. aureus strains for both phases. In several cases, sums of fractional inhibitory concentration values of our test combinations were lower than 0.6, which can be considered as a strong additive interaction. Moreover, composition of CCEO was analysed by gas chromatography-mass spectrometry analysis. In the CCEO, 26 compounds in total were identified, where (E)-cinnamaldehyde was the predominant compound, followed by cinnamyl acetate, alpha-copaene, bornyl acetate and caryophyllene. CONCLUSIONS: Results showed additive in vitro growth-inhibitory effect of CCEO and 8-HQ combination against various standard strains and clinical isolates of S. aureus. SIGNIFICANCE AND IMPACT OF THE STUDY: This is the first report on antibacterial effect of 8-HQ and CCEO combination in liquid and vapour phases. Results of the study suggest these agents as potential candidates for development of new anti-staphylococcal applications that can be used in the inhalation therapy against respiratory infections.
Diamidophosphites from beta-hydroxyamides: readily assembled ligands for Pd-catalyzed asymmetric allylic substitution.[Pubmed:32285048]
Dalton Trans. 2020 May 7;49(17):5625-5635.
Two groups of modular chiral diamidophosphite ligands were easily synthesised from accessible N-Boc-amino alcohols and pseudodipeptides. The reaction of these compounds with [Pd(allyl)Cl]2 in the presence of AgBF4 yielded complexes [Pd(allyl)(L)2]BF4. In addition, metallochelates [Pd(allyl)(L)]BF4 with (S)-methioninol-based P,S-bidentate ligands were prepared. The structures of the novel ligands and complexes were elucidated by means of 2D-NMR and were confirmed by single-crystal X-ray diffraction, as well as by DFT calculations. Asymmetric inducers of this type exhibited high enantioselectivities in the Pd-mediated allylic substitution of (E)-1,3-diphenylallyl ethyl carbonate with CH2(CO2Me)2 (up to 98% ee) and (CH2)4NH (up to 92% ee). Ee values of up to 86% and 73% were obtained in the Pd-catalyzed allylic alkylation of cinnamyl acetate with ethyl 2-oxocyclohexane-1-carboxylate and ethyl 2-oxocyclopentane-1-carboxylate, respectively. The effects of the structural modules, such as the nature of the phosphorus-containing ring or exocyclic substituent, on the catalytic activity and enantioselectivity were investigated.
Chemopreventive and Therapeutic Efficacy of Cinnamomum zeylanicum L. Bark in Experimental Breast Carcinoma: Mechanistic In Vivo and In Vitro Analyses.[Pubmed:32204409]
Molecules. 2020 Mar 19;25(6). pii: molecules25061399.
Comprehensive oncology research suggests an important role of phytochemicals or whole plant foods in the modulation of signaling pathways associated with anticancer action. The goal of this study is to assess the anticancer activities of Cinnamomum zeylanicum L. using rat, mouse, and cell line breast carcinoma models. C. zeylanicum (as bark powder) was administered in the diet at two concentrations of 0.1% (w/w) and 1% (w/w) during the whole experiment in chemically induced rat mammary carcinomas and a syngeneic 4T1 mouse model. After autopsy, histopathological and molecular evaluations of mammary gland tumors in rodents were carried out. Moreover, in vitro analyses using MCF-7 and MDA-MB-231 cells were performed. The dominant metabolites present in the tested C. zeylanicum essential oil (with relative content over 1%) were cinnamaldehyde, cinnamaldehyde dimethyl acetal, cinnamyl acetate, eugenol, linalool, eucalyptol, limonene, o-cymol, and alpha-terpineol. The natural mixture of mentioned molecules demonstrated significant anticancer effects in our study. In the mouse model, C. zeylanicum at a higher dose (1%) significantly decreased tumor volume by 44% when compared to controls. In addition, treated tumors showed a significant dose-dependent decrease in mitotic activity index by 29% (0.1%) and 45.5% (1%) in comparison with the control group. In rats, C. zeylanicum in both doses significantly reduced the tumor incidence by 15.5% and non-significantly suppressed tumor frequency by more than 30% when compared to controls. An evaluation of the mechanism of anticancer action using valid oncological markers showed several positive changes after treatment with C. zeylanicum. Histopathological analysis of treated rat tumor specimens showed a significant decrease in the ratio of high-/low-grade carcinomas compared to controls. In treated rat carcinomas, we found caspase-3 and Bax expression increase. On the other hand, we observed a decrease in Bcl-2, Ki67, VEGF, and CD24 expressions and MDA levels. Assessment of epigenetic changes in rat tumor cells in vivo showed a significant decrease in lysine methylation status of H3K4m3 and H3K9m3 in the high-dose treated group, a dose-dependent increase in H4K16ac levels (H4K20m3 was not changed), down-regulations of miR21 and miR155 in low-dose cinnamon groups (miR22 and miR34a were not modulated), and significant reduction of the methylation status of two out of five gene promoters-ATM and TIMP3 (PITX2, RASSF1, PTEN promoters were not changed). In vitro study confirmed results of animal studies, in that the essential oil of C. zeylanicum displayed significant anticancer efficacy in MCF-7 and MDA-MB-231 cells (using MTS, BrdU, cell cycle, annexin V/PI, caspase-3/7, Bcl-2, PARP, and mitochondrial membrane potential analyses). As a conclusion, C. zeylanicum L. showed chemopreventive and therapeutic activities in animal breast carcinoma models that were also significantly confirmed by mechanistic evaluations in vitro and in vivo.
Biofilm Polysaccharide Display Platform: A Natural, Renewable, and Biocompatible Material for Improved Lipase Performance.[Pubmed:31927950]
J Agric Food Chem. 2020 Feb 5;68(5):1373-1381.
Most of the microorganisms can form biofilms, which makes biofilms an abundant bioresource to be exploited. Due to the limitations of the application of current immobilization methods for biofilms, we developed an immobilization method called the biofilm polysaccharide display (BPD) strategy while maintaining the native biofilm structure and catalytic microenvironment of Clostridium acetobutylicum B3. Lipase Lip181 showed significant improvements in stability after chemical immobilization. For example, immobilized Lip181 retained 74.23% of its original activity after incubation for 14 days, while free Lip181 was totally deactivated. In addition, immobilized Lip181 maintained high residual activity (pH 5.0-11.0), which showed improved resistance to pH changes. Notably, this method did not decrease but slightly increased the relative activity of Lip181 from 6.39 to 6.78 U/mg. Immobilized Lip181 was used to prepare cinnamyl acetate, and it showed a maximum yield of 85.09%. Overall, this biofilm immobilization method may promote the development of biocatalytic and biofilm materials.
A Comparative Analysis of Floral Scent Compounds in Intraspecific Cultivars of Prunus mume with Different Corolla Colours.[Pubmed:31905838]
Molecules. 2019 Dec 30;25(1). pii: molecules25010145.
Prunus mume is the only fragrant flowering species of Prunus. According to the previous studies, benzyl acetate and eugenol dominate its floral scent. However, the diversity of its floral scents remains to be elucidated. In this work, the floral volatiles emitted from eight intraspecific cultivars of P. mume with white, pink and red flowers, were collected and analyzed using headspace solid-phase microextraction combined with gas chromatograms-mass spectrometry (HS-SPME-GC-MS). In total, 31 volatile compounds were identified, in which phenylpropanoids/benzenoids accounted for over 95% of the total emission amounts. Surprisingly, except for benzyl acetate and eugenol, several novel components, such as benzyl alcohol, cinnamyl acohol, cinnamy acetate, and benzyl benzoate were found in some cultivars. The composition of floral volatiles in cultivars with white flowers was similar, in which benzyl acetate was dominant, while within pink flowers, there were differences of floral volatile compositions. Principal component analysis (PCA) showed that the emissions of benzyl alcohol, cinnamyl alcohol, benzyl acetate, eugenol, cinnamyl acetate, and benzyl benzoate could make these intraspecific cultivars distinguishable from each other. Further, hierarchical cluster analysis indicated that cultivars with similar a category and amount of floral compounds were grouped together. Our findings lay a theoretical basis for fragrant plant breeding in P. mume.
Fungicidal Activity of Essential Oils from Cinnamomum cassia against the Pathogenic Fungi of Panax notoginseng Diseases.[Pubmed:31631505]
Chem Biodivers. 2019 Nov;16(11):e1900416.
The frequent disease of Panax notoginseng caused by the pathogenic fungi in field cultivation has become the major threaten to the sustainable development of it. The present study was conducted to find natural agent with potential inhibition against pathogen. Therefore, the inhibitory effects of Cinnamomum cassia (L.) J.Presl essential oils (EOs) against P. notoginseng associated pathogenic fungi were conducted both in vitro and in vivo experiments. The results of the Oxford cup test revealed that C. cassia dry bark EO (50 mg/mL) had significant inhibitory activity on the growth of all tested fungi, and the growth of various pathogens was completely inhibited, except for that of Fusarium solani. Therefore, the constituents of C. cassia EOs were analyzed by GC/MS, and the research demonstrated that the main constituents of C. cassia dry bark EO were trans-cinnamaldehyde (75.65 %), (E)-2-methoxycinnamaldehyde (6.08 %), cinnamaldehyde (3.47 %) and cinnamyl acetate (1.02 %). The MIC results showed that C. cassia dry bark EO and the main compounds had good antifungal effect on the tested strains, and the inhibitory effect was similar to that of hymexazol (chemical pesticide). By analyzing the value of the fraction inhibitory concentration index (FICI), additive effects, irrelevant effects and synergistic effects were observed after the mixture of hymexazol against various pathogens. Moreover, in vivo model showed that C. cassia dry bark EO could reduce the occurrence of anthrax in P. notoginseng. To widen the resources of C. cassia available, the compositions of both C. cassia fresh bark and leaf EOs were also tested and many common compositions existed among them. Taken together, it was concluded that C. cassia EO had the potential use in the field to reduce the pathogenic disease.
A New Natural Antioxidant Biomaterial from Cinnamomum osmophloeum Kanehira Leaves Represses Melanogenesis and Protects against DNA Damage.[Pubmed:31614515]
Antioxidants (Basel). 2019 Oct 11;8(10). pii: antiox8100474.
Cinnamomoum osmophloeum Kanehira (COK) is an indigenous tree species in Taiwan. Chemical compositions, antioxidant activity, mushroom tyrosinase inhibition, melanin synthesis repression, and protection against DNA damage of hydrosol from the COK leaves by steam distillation were examined. We performed 1,1-diphenyl-2-picrylhydrazyl radical scavenging, metal ion chelating, reducing power, and Trolox equivalent antioxidant capacity (TEAC) assays and determined the correlations between total phenolic contents and antioxidant activities. The findings showed that the anti-oxidative properties of COK hydrosol are closely correlated with their phenol contents. Additionally, the major constituents of hydrosol, i.e., cinnamaldehyde and benzaldehyde, had dose-dependent anti-tyrosinase effects against both monophenolase and diphenolase activities. GC-MS analysis revealed that the major bioactive components of hydrosol were trans-cinnamaldehyde (87.7%), benzaldehyde (7.0%), and cinnamyl acetate (5.3%). Moreover, we found that the hydrosol with the presence of benzaldehyde is more potent than pure cinnamaldehyde, and enhances the tyrosinase inhibitory activity of hydrosol. In kinetic analyses, Lineweaver-Burk plots and replots showed that COK hydrosol is a mixed-type inhibitor. Additionally, we found that very low doses of COK hydrosol repressed alpha-melanocyte-stimulating hormone-induced synthesis of microphthalmia-associated transcription factor, leading to decreased melanin synthesis in B16-F10 melanoma cells. These results demonstrated that production of hydrosol from COK leaves using steam distillation may provide a safe and efficacious source of skin-whitening agents for cosmetic and pharmaceutical applications, with antioxidant, anti-tyrosinase, anti-melanogenesis, and DNA protective activities.
Potential of hydrocarbon and oxygenated monoterpenes against Culex pipiens larvae: Toxicity, biochemical, pharmacophore modeling and molecular docking studies.[Pubmed:31378352]
Pestic Biochem Physiol. 2019 Jul;158:156-165.
Culex pipiens is a main vector for Bancroftian filariasis, Rift Valley Fever and diseases caused by other viruses, leaving several peoples with disabilities. In recent years, plant derived compounds have received much attention as potential alternatives to synthetic chemicals due to their low toxicity to mammals and environmental persistence. Twenty-one monoterpenes from different chemical groups (hydrocarbons and oxygenated products) were evaluated against Culex pipiens larvae. In addition, in vivo biochemical studies including effects on acetylcholine esterase (AChE), acid and alkaline phosphatases (ACP and ALP), total adenosine triphosphatase (ATPase) and gamma-aminobutyric acid transaminase (GABA-T) were investigated. Furthermore, in silico studies including pharmacophore elucidation, ADMET analysis and molecular docking of these compounds were performed. Among all tested monoterpenes, hydrocarbons [p-cymene, (R)-(+)-limonene and (+)-alpha-pinene], acetates (cinnamyl acetate, citronellyl acetate, eugenyl acetate and terpinyl acetate), alcohols [(+/-)-beta-citronellol and terpineol], aldehydes [citral and (1R)-(-)-myrtenal] and ketone [(R)-(+)-pulegone] exhibited the highest larval toxicity with LC50=14.88, 27.97, 26.13, 2.62, 3.81, 2.74, 21.65, 1.64, 21.70, 21.76, 1.68 and 1.90mg/L after 48h of exposure, respectively. The compounds proved a significant inhibition of all tested enzymes except total ATPase. The biochemical and molecular docking studies proved that AChE and GABA-T were the main targets for the tested monoterpenes.
Toxicity and effects of essential oils and their components on Dermanyssus gallinae (Acari: Dermanyssidae).[Pubmed:31069572]
Exp Appl Acarol. 2019 May;78(1):65-78.
The acaricidal activity of 30 essential oils against the poultry red mite, Dermanyssus gallinae, female adults and behavioral responses of the mites to these essential oils were investigated. Cinnamon bark oil and clove bud oil showed 100% acaricidal activity after 24 h in the 1.3 mug/m(2) treatment. In addition, four components in cinnamon bark oil and three components in clove bud oil were identified using gas chromatography-mass spectrometry. Cinnamon bark oil showed the highest LD50 value among all of the components, and eugenol showed 0.97-fold higher relative toxicity (RT) than the other components of clove bud oil. The fumigant effects of both essential oils and their seven components were observed using a vapor phase toxicity bioassay. All the substances showed repellent activity except for cinnamyl acetate, which did not show any repellent response even in the > 10 mug treatment. In the experiment using the T-tube olfactometer with the 10 mug treatment of each substance, D. gallinae female adults responded to all the substances except cinnamyl acetate. However, eugenol and eugenol acetate showed an attractant effect after 240 and 120 min of treatment, respectively. These results suggest that the two studied essential oils and their components may be used as control agents against D. gallinae.
P-Chiral 1,7-diphosphanorbornenes: from asymmetric phospha-Diels-Alder reactions towards applications in asymmetric catalysis.[Pubmed:30895990]
Dalton Trans. 2019 Apr 14;48(14):4677-4684.
A straightforward synthesis of P-chiral polycyclic phosphines by an asymmetric Diels-Alder reaction of 1-alkyl-1,2-diphospholes and (5R)-(l-menthyloxy)-2(5H)-furanone (MOxF) is presented. The [4 + 2] cycloaddition reaction of 1,2-diphospholes 1-3 with MOxF (4) proceeded with high diastereoselectivity (de up to 90%) resulting in the corresponding enantiopure anti-endo-1,7-diphosphanorbornenes 5a-7a. The absolute configuration of 5-7 was proved by a variety of 1D/2D NMR correlation methods. The use of the anti-endo-1,7-diphosphanorbornene 5a in the Pd-catalyzed asymmetric allylic alkylation of cinnamyl acetate 8 with cyclic beta-ketoesters 9a,b provided up to 52% ee.
Structural Moieties Required for Cinnamaldehyde-Related Compounds to Inhibit Canonical IL-1beta Secretion.[Pubmed:30544610]
Molecules. 2018 Dec 7;23(12). pii: molecules23123241.
Suppressing canonical NOD-like receptor protein 3 (NLRP3) inflammasome-mediated interleukin (IL)-1beta secretion is a reliable strategy for the development of nutraceutical to prevent chronic inflammatory diseases. This study aimed to find out the functional group responsible for the inhibitory effects of cinnamaldehyde-related compounds on the canonical IL-1beta secretion. To address this, the suppressing capacities of six cinnamaldehyde-related compounds were evaluated and compared by using the lipopolysaccharide (LPS)-primed and adenosine 5'-triphosphate (ATP)-activated macrophages. At concentrations of 25~100 muM, cinnamaldehyde and 2-methoxy cinnamaldehyde dose-dependently inhibited IL-1beta secretion. In contrast, cinnamic acid, cinnamyl acetate, cinnamyl alcohol and alpha-methyl cinnamaldehyde did not exert any inhibition. Furthermore, cinnamaldehyde and 2-methoxy cinnamaldehyde diminished expressions of NLRP3 and pro-IL-1beta. Meanwhile, cinnamaldehyde and 2-methoxy cinnamaldehyde prevented the ATP-induced reduction of cytosolic pro-caspase-1 and increase of secreted caspase-1. In conclusion, for cinnamaldehyde-related compounds to suppress NLRP3 inflammasome-mediated IL-1beta secretion, the propenal group of the side chain was essential, while the substituted group of the aromatic ring played a modifying role. Cinnamaldehyde and 2-methoxy cinnamaldehyde exerted dual abilities to inhibit canonical IL-1beta secretion at both stages of priming and activation. Therefore, there might be potential to serve as complementary supplements for the prevention of chronic inflammatory diseases.
Iridium-Catalyzed Enantioselective and Diastereoselective Allylation of Dioxindoles: A One-Step Synthesis of 3-Allyl-3-hydroxyoxindoles.[Pubmed:30240223]
Org Lett. 2018 Oct 5;20(19):6183-6187.
An iridium-catalyzed asymmetric allylation of dioxindoles, 3-hydroxyoxindoles, regulated by prosthetic groups has been accomplished under mild conditions. The methodology is applicable to a diverse array of 3-hydroxyoxindole and cinnamyl acetate substrates. A range of 3-allyl-3-hydroxyoxindoles containing vicinal tetrasubstituted and trisubstituted stereocenters can be efficiently synthesized in one-step with excellent enantioselectivity (up to >99% enaniomeric excess (ee)) and good diastereoselectivity (up to 11:1 diastereomeric ratio (dr)).
A novel step towards immobilization of biocatalyst using agro waste and its application for ester synthesis.[Pubmed:29733931]
Int J Biol Macromol. 2018 Oct 1;117:366-376.
This work explains the utilization of agro-waste (coconut and peanut shell) to produce mesoporous activated carbon which further utilized as a support material for lipase immobilization (Candida antarctica B, CALB). Various parameters affecting the binding of enzyme to activated carbon with high surface area (1603m(2)g(-1)) were optimized. Maximum 200mugg(-1) CALB has been loaded at 40 degrees C and pH6.8 in 12h by using glutaraldehyde as a cross-linker. The operational parameters such as pH (5.8-8.8) and temperature (30-70 degrees C) were optimized for free and immobilized form of lipase. In thermal stability (50-70 degrees C) study, immobilization of enzyme showed 2.35 folds increased half-life with respect to free enzyme. The samples, before and after immobilization, were characterized by specific surface area, FT-IR, SEM, XRD. This immobilized lipase was successfully used for the synthesis of cinnamyl acetate by transesterification reaction producing 94% conversion in 60min. Catalytic efficiency (58+/-1.08) was seen to be retained for more than five consecutive cycles of chemical reaction for repeated applications. Sequential results towards activity retention were obtained upto 30days of storability study. In the context, this process constitutes a clean route for the development of sustainable biocatalysts from agro waste, capable of applications in various areas.
Pd-Catalyzed Direct C-H Alkenylation and Allylation of Azine N-Oxides.[Pubmed:29629776]
Org Lett. 2018 Apr 20;20(8):2346-2350.
A Pd-catalyzed direct C2-alkenylation of azine N-oxides with allyl acetate is disclosed. The products are formed through an allylation/isomerization cascade process. The use of a tri- tert-butylphosphonium salt as the ligand precursor and KF is mandatory for optimal yields. When cinnamyl acetate is used, the same catalytic system promotes C2-cinnamylation of the azine N-oxide without subsequent isomerization. A mechanism is proposed on the basis of experimental studies and DFT calculations.


