EphedrineCAS# 299-42-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 299-42-3 | SDF | Download SDF |
| PubChem ID | 9294 | Appearance | Powder |
| Formula | C10H15NO | M.Wt | 165.24 |
| Type of Compound | Amines | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
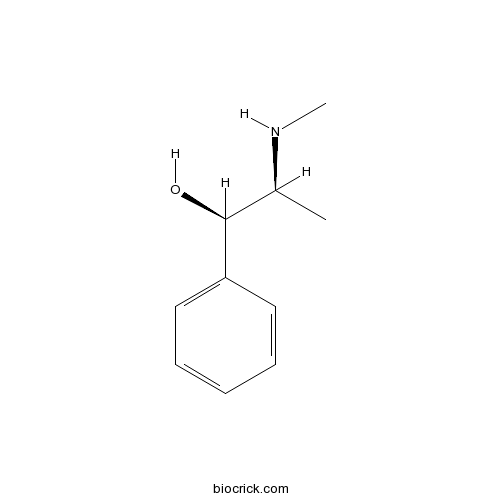
| Chemical Name | (1R,2S)-2-(methylamino)-1-phenylpropan-1-ol | ||
| SMILES | CC(C(C1=CC=CC=C1)O)NC | ||
| Standard InChIKey | KWGRBVOPPLSCSI-WPRPVWTQSA-N | ||
| Standard InChI | InChI=1S/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ephedrine Dilution Calculator

Ephedrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0518 mL | 30.259 mL | 60.518 mL | 121.0361 mL | 151.2951 mL |
| 5 mM | 1.2104 mL | 6.0518 mL | 12.1036 mL | 24.2072 mL | 30.259 mL |
| 10 mM | 0.6052 mL | 3.0259 mL | 6.0518 mL | 12.1036 mL | 15.1295 mL |
| 50 mM | 0.121 mL | 0.6052 mL | 1.2104 mL | 2.4207 mL | 3.0259 mL |
| 100 mM | 0.0605 mL | 0.3026 mL | 0.6052 mL | 1.2104 mL | 1.513 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (5S,6S,7S,8R)-8-Chloro-5,6,7-trihydroxy-2-phenylethyl-5,6,7,8-tetrahydro-4H-chromen-4-one
Catalog No.:BCN9092
CAS No.:626236-06-4
- Bisabolone oxide A
Catalog No.:BCN9091
CAS No.:22567-38-0
- Bisisorhapontigenin E
Catalog No.:BCN9090
CAS No.:
- Delphinidin 3-O-galactoside
Catalog No.:BCN9089
CAS No.:197250-28-5
- (-)-Isobicyclogermacrenal
Catalog No.:BCN9088
CAS No.:73256-82-3
- Cyclohexanecarboxylic acid, 3-[[(2E)-3-[4-(D-glucopyranosyloxy)-3-hydroxyphenyl]-1-oxo-2-propen-1-yl...
Catalog No.:BCN9087
CAS No.:1629852-63-6
- Cucurbitacin Q1
Catalog No.:BCN9086
CAS No.:99530-82-2
- (3r)-7,2'-Dihydroxy-3',4'-dimethoxyisoflavan
Catalog No.:BCN9085
CAS No.:64474-51-7
- (-)-Sesamin
Catalog No.:BCN9084
CAS No.:13079-95-3
- (3β,7β,12β,20Z )- 3,7,12- trihydroxy-11,15,23-trioxo-lanost-8,20-dien-26-oic acid
Catalog No.:BCN9083
CAS No.:1961358-02-0
- Calenduloside E
Catalog No.:BCN9082
CAS No.:26020-14-4
- Chol-8-en-24-oic acid, 7,15-dihydroxy-4,4,14-trimethyl-3,11-dioxo-, (5α)-
Catalog No.:BCN9081
CAS No.:942936-54-1
- Cyanidin 3-O- galactopyranoside
Catalog No.:BCN9094
CAS No.:142506-26-1
- Febrifugine dihydrochloride
Catalog No.:BCN9095
CAS No.:32434-42-7
- Malvidin 3-O-glucoside
Catalog No.:BCN9096
CAS No.:18470-06-9
- Delphinidin 3-O-glucoside
Catalog No.:BCN9097
CAS No.:50986-17-9
- Petunidin 3-O-glucoside
Catalog No.:BCN9098
CAS No.:71991-88-3
- α-Terpinene
Catalog No.:BCN9099
CAS No.:99-86-5
- Cyanidin 3-O-arabinoside
Catalog No.:BCN9100
CAS No.:792868-19-0
- Bisisorhapontigenin F
Catalog No.:BCN9101
CAS No.:
- 3-(2-Hydroxy-4,6-dimethoxyphenyl)-1-(4-hydroxyphenyl)-1-propanone
Catalog No.:BCN9102
CAS No.:151752-07-7
- (3R)-2,3-Dihydro-5,7-dihydroxy-3-[(4-hydroxyphenyl)methyl]-4H-1-benzopyran-4-one
Catalog No.:BCN9103
CAS No.:849727-88-4
- 4H-1-Benzopyran-4-one, 2,3-dihydro-3,5,7-trihydroxy-3-[(4-methoxyphenyl)methyl]-, (R)-
Catalog No.:BCN9104
CAS No.:118204-64-1
- Macrozamin
Catalog No.:BCN9105
CAS No.:6327-93-1
A review of the newly identified impurity profiles in methamphetamine seizures.[Pubmed:32637907]
Forensic Sci Int. 2020 Jun 24;2:194-205.
Forensic intelligence of synthetic illicit drugs suffers a problem of continuous introduction of new synthetic methods, modification of the existing routes of manufacture, and adulterations practiced by criminal networks. Impurity profiling has been indispensable in methamphetamine intelligence based on precursors, synthetic routes, and chemical modifications during trafficking. Law enforcement authorities maintain the credibility and integrity of intelligence information through constant monitoring of the chemical signatures in the illicit drug market. Changes in the synthetic pattern result in new impurity profiles that are important in keeping valuable intelligence information on clandestine laboratories, new synthetic routes, trafficking patterns, and geographical sources of illicit Methamphetamine. This review presents a critical analysis of the methamphetamine impurity profiles and more specifically, profiling based on impurity profiles from Leuckart, Reductive amination, Moscow, Emde, Nagai, Birch, Moscow route; a recent nitrostyrene route and stable isotope signatures. It also highlights the discrimination of Ephedrine from pseudoEphedrine sources and the emerging methamphetamine profiling based on stable isotopes.
Long-term exposure to ephedrine leads to neurotoxicity and neurobehavioral disorders accompanied by up-regulation of CRF in prefrontal cortex and hippocampus in rhesus macaques.[Pubmed:32634541]
Behav Brain Res. 2020 Jul 4:112796.
Drug addiction continues to threaten the health and welfare of people worldwide, and Ephedrine abuse is a serious drug problem in many areas of the world. Ephedrine toxicity is thought to induce behavioral effects primarily through actions on the central nervous system. The corticotropin-releasing factor (CRF) system plays an important role in regulating behavioral effects induced by addictive drugs, but whether CRF is related to Ephedrine toxicity remains unclear. This study seeks to examine whether there is a correlation between the CRF and chronic Ephedrine neurotoxicity. To this end, we established a chronic Ephedrine (0.4-1.6 mg/kg/d) exposure model in rhesus macaques, assessed its effects on body weight and behavior, examined neuronal changes in the prefrontal cortex and hippocampus, and measured the CRF expression in the prefrontal cortex and hippocampus. After 8-weeks of exposure to Ephedrine, the toxic effects of Ephedrine included significant weight loss and induction of behavioral changes in rhesus macaques. In particular, in the modeling group, the abnormal behavioral changes mainly manifested as irritability and behavioral sensitization. Meanwhile, the histological abnormalities included neuronal morphological changes, pyknosis and irregular shapes of neurons in the prefrontal cortex and hippocampus. In addition, the expression levels of CRF mRNA and protein were increased in the prefrontal cortex and hippocampus of Ephedrine-treated animals. In summary, the finding of this study indicated that Ephedrine neurotoxicity can cause neuronal damage in cerebral cortex, which in turn can result in certain neurobehavioral abnormalities, and that CRF expression in prefrontal cortex and hippocampus is elevated in response to Ephedrine exposure. These observations suggested that long-term exposure to Ephedrine might be causing neurotoxicity and leading to neurobehavioral disorders accompanied by up-regulation of CRF in prefrontal cortex and hippocampus.
A fast and simple approach for the quantification of five anti-hypersensitivity drugs in saliva and urine by portable ion mobility spectrometry based on magnetic graphene oxide dispersive solid phase extraction.[Pubmed:32629193]
J Pharm Biomed Anal. 2020 Jun 10;189:113414.
Fatal road traffic crashes are often related to multifarious risk factors, among which driving under the influence of drugs (DUID) has been reported as a significantly contributing cause. The first worry about the side-effect of influencing driving drugs is central nervous system adverse reaction, and anti-hypersensitivity drugs are a class of drugs with such side effects meanwhile has been widely used for common allergic diseases thus posing a great challenge to road safety and demanding a rapid and efficient method to detect. In this work, a method based on magnetic graphene oxide dispersive solid phase extraction (MGO-D-SPE) combined with ion mobility spectrometry (IMS) was firstly introduced for simultaneous determination of Ephedrine, pseudoEphedrine, diphenhydramine, promethazine and terfenadine in saliva and urine matrices. The prepared MGO was characterized by Fourier transform infrared (FT-IR) spectroscopy and thermo gravimetric analysis (TGA). Various parameters affecting extraction efficiency as well as instrumental acquisition sensitivity were studied and optimized. Under the optimum experimental conditions, the method was fully validated and the results demonstrated that the proposed method exhibited some advantages, including a good linearity covering large concentration ranges of 51.0-3040 ng ml(-1) for five anti-hypersensitivity drugs, and good accuracy was also obtained with high precision (CV% < 5.0 %). LODs and LOQs were 10.2-50.4 ng.mL(-1) and 30.6-101.3 ng.mL(-1), respectively. Consequently, the MGO-D-SPE-IMS methodology succeeded in building a hitherto unexplored tool for quantifying anti-hypersensitivity drugs in saliva and urine matrices of interest in DUID research field.
Techniques for preventing hypotension during spinal anaesthesia for caesarean section.[Pubmed:32619039]
Cochrane Database Syst Rev. 2020 Jul 1;7:CD002251.
BACKGROUND: Maternal hypotension is the most frequent complication of spinal anaesthesia for caesarean section. It can be associated with nausea or vomiting and may pose serious risks to the mother (unconsciousness, pulmonary aspiration) and baby (hypoxia, acidosis, neurological injury). OBJECTIVES: To assess the effects of prophylactic interventions for hypotension following spinal anaesthesia for caesarean section. SEARCH METHODS: We searched Cochrane Pregnancy and Childbirth's Trials Register (9 August 2016) and reference lists of retrieved studies. SELECTION CRITERIA: Randomised controlled trials, including full texts and abstracts, comparing interventions to prevent hypotension with placebo or alternative treatment in women having spinal anaesthesia for caesarean section. We excluded studies if hypotension was not an outcome measure. DATA COLLECTION AND ANALYSIS: Two review authors independently assessed study quality and extracted data from eligible studies. We report 'Summary of findings' tables using GRADE. MAIN RESULTS: We included 125 studies involving 9469 women. Interventions were to prevent maternal hypotension following spinal anaesthesia only, and we excluded any interventions considered active treatment. All the included studies reported the review's primary outcome. Across 49 comparisons, we identified three intervention groups: intravenous fluids, pharmacological interventions, and physical interventions. Authors reported no serious adverse effects with any of the interventions investigated. Most trials reported hypotension requiring intervention and Apgar score of less than 8 at five minutes as the only outcomes. None of the trials included in the comparisons we describe reported admission to neonatal intensive care unit. Crystalloid versus control (no fluids) Fewer women experienced hypotension in the crystalloid group compared with no fluids (average risk ratio (RR) 0.84, 95% confidence interval (CI) 0.72 to 0.98; 370 women; 5 studies; low-quality evidence). There was no clear difference between groups in numbers of women with nausea and vomiting (average RR 0.19, 95% CI 0.01 to 3.91; 1 study; 69 women; very low-quality evidence). No baby had an Apgar score of less than 8 at five minutes in either group (60 babies, low-quality evidence). Colloid versus crystalloid Fewer women experienced hypotension in the colloid group compared with the crystalloid group (average RR 0.69, 95% CI 0.58 to 0.81; 2009 women; 27 studies; very low-quality evidence). There were no clear differences between groups for maternal hypertension requiring intervention (average RR 0.64, 95% CI 0.09 to 4.46, 3 studies, 327 women; very low-quality evidence), maternal bradycardia requiring intervention (average RR 0.98, 95% CI 0.54 to 1.78, 5 studies, 413 women; very low-quality evidence), nausea and/or vomiting (average RR 0.89, 95% CI 0.66 to 1.19, 14 studies, 1058 women, I(2) = 29%; very low-quality evidence), neonatal acidosis (average RR 0.83, 95% CI 0.15 to 4.52, 6 studies, 678 babies; very low-quality evidence), or Apgar score of less than 8 at five minutes (average RR 0.24, 95% CI 0.03 to 2.05, 10 studies, 730 babies; very low-quality evidence). Ephedrine versus phenylephrine There were no clear differences between Ephedrine and phenylephrine groups for preventing maternal hypotension (average RR 0.92, 95% CI 0.71 to 1.18; 401 women; 8 studies; very low-quality evidence) or hypertension (average RR 1.72, 95% CI 0.71 to 4.16, 2 studies, 118 women, low-quality evidence). Rates of bradycardia were lower in the Ephedrine group (average RR 0.37, 95% CI 0.21 to 0.64, 5 studies, 304 women, low-quality evidence). There was no clear difference in the number of women with nausea and/or vomiting (average RR 0.76, 95% CI 0.39 to 1.49, 4 studies, 204 women, I(2) = 37%, very low-quality evidence), or babies with neonatal acidosis (average RR 0.89, 95% CI 0.07 to 12.00, 3 studies, 175 babies, low-quality evidence). No baby had an Apgar score of less than 8 at five minutes in either group (321 babies; low-quality evidence). Ondansetron versus control Ondansetron administration was more effective than control (placebo saline) for preventing hypotension requiring treatment (average RR 0.67, 95% CI 0.54 to 0.83; 740 women, 8 studies, low-quality evidence), bradycardia requiring treatment (average RR 0.49, 95% CI 0.28 to 0.87; 740 women, 8 studies, low-quality evidence), and nausea and/or vomiting (average RR 0.35, 95% CI 0.24 to 0.51; 653 women, 7 studies, low-quality evidence). There was no clear difference between the groups in rates of neonatal acidosis (average RR 0.48, 95% CI 0.05 to 5.09; 134 babies; 2 studies, low-quality evidence) or Apgar scores of less than 8 at five minutes (284 babies, low-quality evidence). Lower limb compression versus control Lower limb compression was more effective than control for preventing hypotension (average RR 0.61, 95% CI 0.47 to 0.78, 11 studies, 705 women, I(2) = 65%, very low-quality evidence). There was no clear difference between the groups in rates of bradycardia (RR 0.63, 95% CI 0.11 to 3.56, 1 study, 74 women, very low-quality evidence) or nausea and/or vomiting (average RR 0.42, 95% CI 0.14 to 1.27, 4 studies, 276 women, I(2) = 32%, very-low quality evidence). No baby had an Apgar score of less than 8 at five minutes in either group (130 babies, very low-quality evidence). Walking versus lying There was no clear difference between the groups for women with hypotension requiring treatment (RR 0.71, 95% CI 0.41 to 1.21, 1 study, 37 women, very low-quality evidence). Many included studies reported little to no information that would allow an assessment of their risk of bias, limiting our ability to draw meaningful conclusions. GRADE assessments of the quality of evidence ranged from very low to low. We downgraded evidence for limitations in study design, imprecision, and indirectness; most studies assessed only women scheduled for elective caesarean sections. External validity also needs consideration. Readers should question the use of colloids in this context given the serious potential side effects such as allergy and renal failure associated with their administration. AUTHORS' CONCLUSIONS: While interventions such as crystalloids, colloids, Ephedrine, phenylephrine, ondansetron, or lower leg compression can reduce the incidence of hypotension, none have been shown to eliminate the need to treat maternal hypotension in some women. We cannot draw any conclusions regarding rare adverse effects associated with use of the interventions (for example colloids) due to the relatively small numbers of women studied.
Methylephedrine-induced heart failure in a habitual user of paediatric cough syrup: a case report.[Pubmed:32617471]
Eur Heart J Case Rep. 2020 Apr 27;4(3):1-4.
Background: For relief of cold symptoms, methylEphedrine is considered to be safer than Ephedrine, particularly when used at the predetermined dose. It is often present in various over-the-counter (OTC) drugs for cold, including paediatric cough syrups. Case summary: A 52-year-old man presented with worsening dyspnoea and anorexia for 2 weeks. He was a night shift worker and had been habitually taking large doses of methylEphedrine-containing paediatric cough syrup for 20 years for sleep averting. On admission, his chest X-ray revealed pulmonary congestion and electrocardiogram showed sinus tachycardia with left-axis deviation. Echocardiography revealed diffuse hypokinesis with a reduced ejection fraction (EF) of 25%. The B-type natriuretic peptide level was elevated to 1092 ng/L. Even after treatment with low-dose dobutamine and furosemide in intensive care unit, right-heart catheterization demonstrated a 'wet and cold' profile. Coronary angiography revealed normal coronary arteries. Pathological examination by endomyocardial biopsy revealed cardiomyocyte hypertrophy with moderate interstitial and replacement fibrosis. In addition, cardiac magnetic resonance imaging revealed diffuse hypokinesis with mid-wall late gadolinium enhancement, which suggested fibrosis. Discontinuation of the cough syrup and optimal medical treatment with an angiotensin-converting enzyme inhibitor and a beta blocker resulted in improvement in the heart failure symptoms to New York Class Association Class II. The EF also improved to 50% at 4 months after discharge. Discussion: MethylEphedrine is considered to have adrenergic effects; it has milder side effects on the cardiovascular system than Ephedrine. However, the long-lasting excessive intake of methylEphedrine, even through OTC paediatric cough syrups, has the potential to cause heart failure.
Ephedrine causes retinal damage in SD rats associating with JAK2/STAT3 pathway.[Pubmed:32602374]
Cutan Ocul Toxicol. 2020 Jun 30:1-15.
Ephedrine has various side effects in the cardiovascular and nervous systems. However, the cellular mechanism of toxicity remains unknown, specifically on the retina. This study was to investigate effects of Ephedrine on the retina and explore the underlying mechanisms. Sprague Dawley rats were treated with Ephedrine (n = 10) or saline (n = 10) by oral gavage for seven days. The retinal morphology was evaluated by Toluidine blue staining. Apoptosis-related markers were detected in the retinal lysate. Enzyme-linked immunosorbent assays were used to measure neurotransmitters and oxidative stress markers. Real-time PCR and western blot were used to measure gene and protein expression, respectively. Our results demonstrated that Ephedrine induced apoptosis in the retina, increased dopamine level as well as oxidative stress, and down-regulated the Jak2/Stat3 gene expression as well as protein expression of p-JAK2/p-STAT3. Our study indicated that Ephedrine treatment caused retinal damage in SD rats, which may be associated with the JAK2/STAT3 pathway.
Comparison of four documents describing adrenaline purification, and the work of three important scientists, Keizo Uenaka, Nagai Nagayoshi and Jokichi Takamine.[Pubmed:32593376]
J Anesth Hist. 2020 Jun;6(2):42-48.
The name of Keizo Uenaka has not been documented in textbooks. However, Uenaka was the scientist who worked on Ephedrine and played a practical role in the purification and crystallization of adrenaline. His handwritten memorandum, "On Adrenaline, Memorandum, July to December, 1900" is now stored in a Buddhist temple, Kyougyou-ji in Nashio, Japan. In the present report, we compared Uenaka's original description and Jokichi Takamine's published scientific reports, and examined how each statement in four documents are related to each other in terms of successful adrenaline crystallization. Uenaka's memorandum contained precise procedures and experimental tips for successful purification. The experimental steps were considered to transcribed in the first published document in The American Journal of Pharmacy by Takamine in 1901, and summarized in another document in ``Journal of Physiology'' in 1901. A Japanese version was published in ``Yakugakuzasshi'' in 1903 by translating the English paper in the American Journal of Pharmacy published in 1901. Reading Uenaka's memorandum, we realized that he tirelessly and diligently undertook routine experiments that to some of us might seem boring and laborious. Although the name of Uenaka is not globally well known, he was the main scientist who did the actual work of purifying adrenaline.
Application of a chiral HPLC-MS/MS method for the determination of 13 related amphetamine-type stimulants to forensic samples: Interpretative hypotheses.[Pubmed:32589765]
Drug Test Anal. 2020 Jun 26.
Interpretation of amphetamine-type stimulant (ATS) findings in urine samples can be challenging without chiral information. We present a sensitive enantioselective high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method for the quantification of (R)-amphetamine, (S)-amphetamine, (R)-methamphetamine, (S)-methamphetamine, (1R,2R)-pseudoEphedrine, (1S,2S)-pseudoEphedrine, (1R,2S)-Ephedrine, (1S,2R)-Ephedrine, (1R,2S)-norEphedrine, (1S,2R)-norEphedrine, (R)-cathinone, (S)-cathinone, and (1S,2S)-norpseudoEphedrine (cathine) in urine. The method was successfully applied to more than 100 authentic urine samples from forensic casework. Additionally, samples from a controlled self-administration of (1S,2S)-pseudoEphedrine (Rinoral(R), 1,200 mg within six days) were analyzed. The results strengthen the hypothesis that (1R,2S)-norEphedrine is a minor metabolite of amphetamine and methamphetamine. We suggest cathine and (1S,2R)-norEphedrine as minor metabolites of amphetamine racemate in humans. Small methamphetamine concentrations detected in samples with high concentrations of amphetamine could result from a metabolic formation by methylation of amphetamine, although in samples with an (R)/(S) ratio for methamphetamine < 1 an additional (previous) (S)-methamphetamine consumption seems likely. Our data suggest that even amphetamine concentrations exceeding methamphetamine concentrations in urine can be caused by biotransformation of methamphetamine to amphetamine as long as no (R)-amphetamine is detected. However, without chiral information such findings might be (falsely) assumed as a co-consumption of both substances. Cathinone enantiomers detected in urine samples with high amphetamine concentrations can be interpreted as metabolites of amphetamine. Additionally, the results of the self-administration study revealed that both cathinone enantiomers are minor metabolites of (1S,2S)-pseudoEphedrine which is the active ingredient of various cold medicines. Enantioselective analysis is a powerful tool to avoid misinterpretation of ATS findings in urine samples.
Efficacy of Dexmedetomidine vs Morphine as an Adjunct in a Paravertebral Block with Bupivacaine in Postoperative Analgesia Following Modified Radical Mastectomy.[Pubmed:32582491]
Cureus. 2020 May 22;12(5):e8231.
Objective To observe the efficacy of dexmedetomidine vs morphine as an adjunct in a paravertebral block (PVB) with bupivacaine in postoperative analgesia following modified radical mastectomy. Study design This was a randomized controlled trial performed from June 2018 to August 2019 in the Department of Anesthesia, Bakhtawar Amin Medical and Dental College, Ch. Pervaiz Ellahi Institute of Cardiology, Multan, Gurki Hospital, Services Institute of Medical Sciences, and Sheikh Zayed Hospital, Lahore. Methodology Seventy-eight patients were equally divided into group M, which received morphine (3 mg) and group D, which received dexmedetomidine (1 microg/kg), along with 20 cc 0.25% bupivacaine, for PVB. The primary outcome included morphine requirements in the post-anesthesia care unit (PACU). Secondary outcomes included the quality and duration of analgesia, intraoperative doses of fentanyl and propofol, postoperative doses of diclofenac required, postoperative nausea and vomiting (PONV), and the Ramsey sedation score. Data were entered into SPSS version 23 (IBM Corp., Armonk, NY) and analyzed by applying the independent t-test, Mann Whitney U-test, and the chi-square test or Fischer's exact test, as appropriate. PEphedrine and morphine used were higher in group D (p-value 0.033 and 0.013, respectively). In the PACU, 33.3% of group D patients as compared to 12.8% of group M patients needed morphine (p=0.032). Postoperatively, diclofenac consumption was higher in group D (p<0.001). Postoperative pain was lower and sedation was higher in group M (p<0.05). Conclusion As an adjunct to bupivacaine in PVB for MRM, morphine is superior to dexmedetomidine.
Control of Spinal Anesthesia-Induced Hypotension in Adults.[Pubmed:32581577]
Local Reg Anesth. 2020 Jun 3;13:39-46.
Spinal anesthesia-induced hypotension (SAIH) occurs frequently, particularly in the elderly and in patients undergoing caesarean section. SAIH is caused by arterial and venous vasodilatation resulting from the sympathetic block along with a paradoxical activation of cardioinhibitory receptors. Bradycardia after spinal anesthesia (SA) must always be treated as a warning sign of an important hemodynamic compromise. Fluid preloading (before initiation of the SA) with colloids such as hydroxyethyl starch (HES) effectively reduces the incidence and severity of arterial hypotension, whereas crystalloid preloading is not indicated. Co-loading with crystalloid or colloid is as equally effective to HES preloading, provided that the speed of administration is adequate (ie, bolus over 5 to 10 minutes). Ephedrine has traditionally been considered the vasoconstrictor of choice, especially for use during SAIH associated with bradycardia. Phenylephrine, a alpha1 adrenergic receptor agonist, is increasingly used to treat SAIH and its prophylactic administration (ie, immediately after intrathecal injection of local anesthetics) has been shown to decrease the incidence of arterial hypotension. The role of norepinephrine as a possible alternative to phenylephrine seems promising. Other drugs, such as serotonin receptor antagonists (ondansetron), have been shown to limit the blood pressure drop after SA by inhibiting the Bezold-Jarisch reflex (BJR), but further studies are needed before their widespread use can be recommended.
Identification of Active Compounds of Mahuang Fuzi Xixin Decoction and Their Mechanisms of Action by LC-MS/MS and Network Pharmacology.[Pubmed:32565854]
Evid Based Complement Alternat Med. 2020 May 23;2020:3812180.
The decoction is an important dosage form of traditional Chinese medicine (TCM) administration. The Mahuang Fuzi Xixin decoction (MFXD) is widely used to treat allergic rhinitis (AR) in China. However, its active compounds and therapeutic mechanisms are unclear. The aim of this study was to establish an integrative method to identify the bioactive compounds and reveal the mechanisms of action of MFXD. LC-MS/MS was used to identify the compounds in MFXD, followed by screening for oral bioavailability. TCMSP, BindingDB, STRING, DAVID, and KEGG databases and algorithms were used to gather information. Cytoscape was used to visualize the networks. Twenty-four bioactive compounds were identified, and thirty-seven predicted targets of these compounds were associated with AR. DAVID analysis suggested that these compounds exert their therapeutic effects by modulating the Fc epsilon RI, B-cell receptor, Toll-like receptor, TNF, NF-kappaB, and T-cell receptor signaling pathways. The PI3K/AKT and cAMP signaling pathways were also implicated. Ten of the identified compounds, quercetin, pseudoEphedrine, Ephedrine, beta-asarone, methylEphedrine, alpha-linolenic acid, cathine, ferulic acid, nardosinone, and higenamine, seemed to account for most of the beneficial effects of MFXD in AR. This study showed that LC-MS/MS followed by network pharmacology analysis is useful to elucidate the complex mechanisms of action of TCM formulas.
[Hypotension - a complication of subarachnoid anesthesia especially dangerous in patients aged].[Pubmed:32564050]
Pol Merkur Lekarski. 2020 Jun 17;48(285):215-220.
Demographic data clearly show the progressive aging of societies. Problems and specificity of anaesthesia in the elderly becomes a particularly important issue in this context. Spinal anesthesia is a method often used to anesthetize elderly patients, and hypotension is one of its most common early complications. Untreated or improperly treated hypotension increases the risk of perioperative complications such as myocardial infarction, ischemic stroke or acute renal failure. The prevention of hypotension consists of intravenous fluid therapy and pre-emptive use of vasoconstrictor drugs. Among vasoconstrictors, Ephedrine and phenylephrine are commonly used to treat hypotension due to spinal anaesthesia. Both drugs are available in Poland. Issues related to their use in the treatment of hypotension caused by spinal anaesthesia in the elderly, including the features of both drugs, their method of administration and dosage based on the literature and own experience are the subject of this study. It should be noted, however, that most studies in the use of Ephedrine and phenylephrine as a targeted treatment for hypotension concern the obstetric patient population while the elderly population is underrepresented and the results are inconclusive.
Aqueous chlorination of ephedrine: Kinetic, reaction mechanism and toxicity assessment.[Pubmed:32563881]
Sci Total Environ. 2020 Jun 12;740:140146.
Ephedrine (EPH) is widely detected in the water environment, because it is the major ingredient in drugs treating influenza, asthma or hypotension, and is also a highly sought-after chemical precursor in the illicit manufacture of methamphetamine. In this study, transformation of EPH during the chlorination process was investigated for the first time, and the impact of water parameters including pH, different cations and anions on EPH transformation was evaluated as well. The degradation of EPH in the presence of NaClO fit the second order reaction kinetics, with a rate constant of 7.43 x 10(2) ((mol.L(-1))(-1).min(-1)). Increasing the dosage of NaClO increased the observed pseudo first order rate constant for EPH degradation (kobs). Degradation rate of EPH decreased with the increasing pH from 2.0 to 10.0, due to the formation of a chlorammonium intermediate that reacted with NaClO. Low concentration of Br(-) and I(-) did not exert significant influence on the degradation of EPH, while at high concentrations a promotive effect was observed. Other ions including Fe(3+), Cu(2+), NO3(-), SO4(2-), Mg(2+) and Ca(2+) exerted negative effects even at relatively low concentrations. Based on the degradation products/intermediates identified by UPLC-MS/MS, the EPH degradation pathways were proposed. The reaction mechanism involved in the EPH degradation included dehydration, hydroxylation, deamination and demethylation. Toxicity assays by V. qinghaiensis sp. nov proved that the EPH transformation products were much more toxic than the parent compound. Results indicated that chlorination is an effective approach for the elimination of EPH in the aquatic environment, however, attention should be paid to its toxicity involvement during the chlorination process.
Dural sac cross-sectional area is a highly effective parameter for spinal anesthesia in geriatric patients undergoing transurethral resection of the prostate: a prospective, double blinded, randomized study.[Pubmed:32493211]
BMC Anesthesiol. 2020 Jun 3;20(1):139.
BACKGROUND: Spinal anesthesia is optimal choice for transurethral resection of the prostate (TURP), but the sensory block should not cross the T10 level. With advancing age, the sensory blockade level increases after spinal injection in some patients with spinal canal stenosis. We optimize the dose of spinal anesthesia according to the decreased ratio of the dural sac cross-sectional area (DSCSA), the purpose of this study is to hypothesis that if DSCSA is an effective parameter to modify the dosage of spinal anesthetics to achieve a T10 blockade in geriatric patients undergoing TURP. METHODS: Sixty geriatric patients schedule for TURP surgery were enrolled in this study. All subjects were randomized divided into two groups, the ultrasound (group U) and the control (group C) groups, patient receive either a dose of 2 ml of 0.5% isobaric bupivacaine in group C, or a modified dose of 0.5% isobaric bupivacaine in group U. We measured the sagittal anteroposterior diameter (D) of the dural sac at the L3-4 level with ultrasound, and calculated the approximate DSCSA (A) according to the following formula: A = pi(D/2)(2), ( pi = 3.14). The modified dosage of bupivacaine was adjusted according to the decreased ratio of the DSCSA. RESULTS: The cephalad spread of the sensory blockade level was significantly lower (P < 0.001) in group U (T10, range T7-T12) compared with group C (T3, range T2-T9). The dosage of bupivacaine was significantly decreased in group U compared with group C (P < 0.001). The regression times of the two segments were delay in group U compared with group C (P < 0.001). The maximal decrease in MAP was significantly higher in the group C than in group U after spinal injection (P < 0.001), without any modifications HR in either group. Eight patients in group C and two patients in group U required Ephedrine (P = 0.038). CONCLUSIONS: The DSCSA is a highly effective parameter for spinal anesthesia in geriatric patients undergoing TURP, a modified dose of local anesthetic is a critical factor for controlling the sensory level. TRIAL REGISTRATION: This study was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR1800015566).on 8, April, 2018.


