CoryneineCAS# 7224-66-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7224-66-0 | SDF | Download SDF |
| PubChem ID | 165581 | Appearance | Powder |
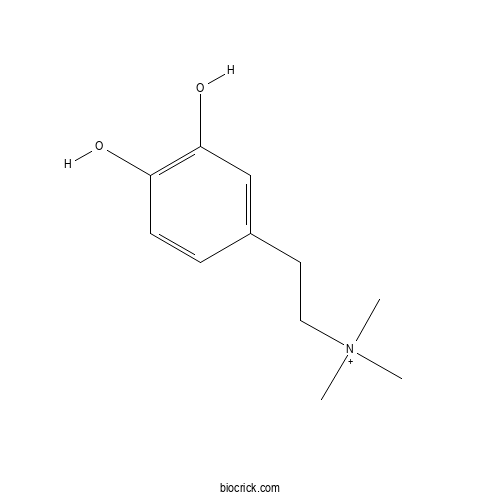
| Formula | C11H18NO2 | M.Wt | 196.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)ethyl-trimethylazanium | ||
| SMILES | C[N+](C)(C)CCC1=CC(=C(C=C1)O)O | ||
| Standard InChIKey | VDTBORSEFUUDTP-UHFFFAOYSA-O | ||
| Standard InChI | InChI=1S/C11H17NO2/c1-12(2,3)7-6-9-4-5-10(13)11(14)8-9/h4-5,8H,6-7H2,1-3H3,(H-,13,14)/p+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Coryneine Dilution Calculator

Coryneine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0942 mL | 25.4712 mL | 50.9424 mL | 101.8849 mL | 127.3561 mL |
| 5 mM | 1.0188 mL | 5.0942 mL | 10.1885 mL | 20.377 mL | 25.4712 mL |
| 10 mM | 0.5094 mL | 2.5471 mL | 5.0942 mL | 10.1885 mL | 12.7356 mL |
| 50 mM | 0.1019 mL | 0.5094 mL | 1.0188 mL | 2.0377 mL | 2.5471 mL |
| 100 mM | 0.0509 mL | 0.2547 mL | 0.5094 mL | 1.0188 mL | 1.2736 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3',5-Di-O-methyl quercetin
Catalog No.:BCN0218
CAS No.:40554-94-7
- 3,5,6,7,8,4'-hexamethoxyflavone
Catalog No.:BCN0217
CAS No.:34170-18-8
- Rutarensin
Catalog No.:BCN0216
CAS No.:119179-04-3
- 4-Methyl-6-phenyl-2H-pyranone
Catalog No.:BCN0215
CAS No.:4467-30-5
- Physalin F
Catalog No.:BCN0214
CAS No.:57423-71-9
- 3-Oxo-olean-12-en-28-oic acid methyl ester
Catalog No.:BCN0213
CAS No.:1721-58-0
- 7-Ketocholesterol
Catalog No.:BCN0212
CAS No.:566-28-9
- 4',5-Di-O-methyl quercetin
Catalog No.:BCN0211
CAS No.:100648-56-4
- Lathyranoic acid A
Catalog No.:BCN0210
CAS No.:850560-44-0
- Isophysalin G
Catalog No.:BCN0209
CAS No.:152221-21-1
- (3beta,22alpha)-26-(beta-glucopyranosyloxy)-22-methoxyfurost-5-en-3-yl 2-O-(6-deoxy-alpha-mannopyranosyl)-beta-glucopyranosiduronic acid
Catalog No.:BCN0208
CAS No.:107783-53-9
- 9,11-Dehydro-beta-boswellic acid
Catalog No.:BCN0207
CAS No.:471-65-8
- Corysamine chloride
Catalog No.:BCN0220
CAS No.:11028-77-6
- Tubuloside B
Catalog No.:BCN0221
CAS No.:112516-04-8
- Linaroside
Catalog No.:BCN0222
CAS No.:53452-12-3
- 6-O-Methylcatalpol
Catalog No.:BCN0223
CAS No.:1617-84-1
- Ricinine
Catalog No.:BCN0224
CAS No.:524-40-3
- Quercetin 3-O-malonylglucoside
Catalog No.:BCN0225
CAS No.:96862-01-0
- Luteolin-7-O-alpha-L-arabinopyranosyl (1->6)-beta-D-glucopyranoside
Catalog No.:BCN0226
CAS No.:52714-82-6
- Emodin 6-O-beta-D-glucoside
Catalog No.:BCN0227
CAS No.:34298-85-6
- 24-Methylene cycloartanyl ferulate
Catalog No.:BCN0228
CAS No.:469-36-3
- Valtrate Hydrine B4
Catalog No.:BCN0229
CAS No.:18296-48-5
- Polygalasaponin II
Catalog No.:BCN0230
CAS No.:162857-62-7
- L-Gamma-Glutamyl-S-[(4-hydroxyphenyl)methyl]-L-cysteinylglycine
Catalog No.:BCN0231
CAS No.:129636-38-0
Counter effects of higenamine and coryneine, components of aconite root, on acetylcholine release from motor nerve terminal in mice.[Pubmed:11256693]
J Asian Nat Prod Res. 2000;2(3):195-203.
The counter effects of higenamine and Coryneine, components of aconite root, on acetylcholine (ACh) release from motor nerve terminals in the mouse phrenic nerve-diaphragm muscle preparation were studied by a radioisotope method. Both nerve-evoked release and spontaneous release of [3H]-ACh from the preparation preloaded with [3H]-choline were measured. The change in the tetanic tension of muscle was simultaneously recorded in the same preparation. Higenamine (10 microM) augmented both the nerve-evoked and spontaneous ACh releases, and the muscle tension. The effects were inhibited by pretreatment with propranolol (10 microM), a beta-adrenoceptor antagonist. Coryneine reduced the nerve-evoked release of ACh, accelerated the decay of tetanic tension (tetanic fade) at 30 microM, and it depressed the peak amplitude of tetanic tension at a higher concentration of 100 microM. These results suggest that of the two components contained in aconite root, higenamine increases ACh release via activation of beta-adrenoceptor, and conversely Coryneine depresses ACh release by preferentially acting at motor nerve terminal.
Depolarizing neuromuscular blocking action of coryneine derived from aconite root in isolated mouse phrenic nerve-diaphragm muscles.[Pubmed:7492984]
Biol Pharm Bull. 1995 May;18(5):691-5.
The mode of the neuromuscular blocking action of Coryneine (a quaternary ammonium derivative of dopamine) derived from aconite root was investigated in isolated phrenic nerve-diaphragm muscles and denervated diaphragm muscles of mice. Coryneine (20-150 microM) blocked the nerve-evoked twitch response without affecting the contraction evoked by electrical stimulation of the muscle. The blocking effect was reversed by neostigmine, a cholinesterase inhibitor. The electrical charge-response curve on depolarization produced by iontophoretically applied acetylcholine (ACh) at the endplate regions in normal muscles was shifted to the right on decreasing the maximal response by 40 microM Coryneine. The double-reciprocal plot revealed that Coryneine reduced the apparent affinity of ACh for its receptor on decreasing the maximal response. Coryneine (20 microM-2mM) itself depolarized the endplate membrane and this effect was reversibly suppressed by 1 and 5 microM pancuronium. Coryneine 30 microM-10mM) produced contractions of denervated muscles in a concentration-dependent manner and the effects were reduced by 70nM pancuronium. These results indicate that Coryneine is a depolarizing agent and a mixed-type competitive and noncompetitive neuromuscular blocker.
Relations between structure and nicotine-like activity: X-ray crystal structure analysis of (-)-cytisine and (-)-lobeline hydrochloride and a comparison with (-)-nicotine and other nicotine-like compounds.[Pubmed:2590771]
Br J Pharmacol. 1989 Nov;98(3):799-808.
1. Although (-)-cytisine is a rigid structure, it occurs in the crystal in two distinct but very similar conformations in which the pyridone ring is tilted relative to the charged nitrogen atom at much the same angle as the pyridine ring is in (-)-nicotine hydrogen iodide. The carbonyl group in the pyridone ring of (-)-cytisine, however, is on the side of the ring opposite to pyridine nitrogen in (-)-nicotine. 2. The pKa of (-)-lobeline HCl at 25 degrees C is 8.6 (approx), indicating that (-)-lobeline is at least 90% in the protonated form at physiological pH (7.6). It is probably the phenyl 2-keto-ethyl part of (-)-lobeline, rather than the phenyl 2-hydroxy-ethyl part, which interacts with the receptor. 3. The combination within one molecule of a charged ('onium') nitrogen atom lying out of the plane of, and some distance (4.5-6.5 A) from, an aromatic ring is common to many compounds with nicotine-like activity (e.g. nicotine, cytisine, choline phenyl ether bromide, dimethyl-phenyl-piperazinium (DMPP) iodide, Coryneine iodide and m-hydroxyphenylpropyl trimethyl ammonium iodide). In some molecules the aromatic ring can be replaced by an unsaturated group, such as carbonyl (e.g. acetylcholine) or double-bonds (e.g. anatoxin). 4. Activity at nicotinic receptors appears to involve interactions between the positively charged nitrogen atom and a negatively charged group, probably close to cysteine residues 192 and 193 in the receptor. It is suggested that rather than specific groups in the molecule also being involved, activity at nicotinic receptors depends on interactions between a flat part of the drug containing double-bonds, or systems of double bonds, and a planar area in the receptor, possibly tyrosine or phenylalanine residues.
Hypaconitine, the dominant constituent responsible for the neuromuscular blocking action of the Japanese-sino medicine "bushi" (aconite root).[Pubmed:3210453]
Jpn J Pharmacol. 1988 Oct;48(2):290-3.
The neuromuscular blocking actions of several constituents extracted from Japanese-sino medicine, aconite, were compared in mouse phrenic nerve-diaphragm muscle preparations. Hypaconitine (HAT) was more potent than aconitine (ATN), mesaconitine (MAT) and deoxyaconitine. Lipohypaconitine, Coryneine and lipodeoxyaconitine were less effective. Lipoaconitine, benzoylmesaconine, higenamine, kobusine and chasmanine were not effective. The blockades by HAT, ATN and MAT were not recovered by neostigmine. The mechanisms of blockade were similar to that of aconite crude extract. These results suggest that aconite action is dependent on HAT, a main constituent.
A comparison of cinobufotenine (the quaternary derivative of 5-HT) and some related compounds with coryneine (the quaternary derivative of dopamine) on the frog rectus, guinea-pig ileum and rat fundus strip preparations.[Pubmed:6449223]
Br J Pharmacol. 1980 Aug;69(4):597-600.
1 Coryneine is 2.7 times as active as cinobufotenine on the frog rectus but on the guinea-pig ileum cinobufotenine is 1.5 times as active as Coryneine. Cinobufotenine is a potent stimulant of parasympathetic ganglia and its effect are competitively antagonized by hexamethonium. 2 The effects of pH on activity relative to a standard whose ionisation is constant (Me4+N or the trimethylammonium analogue of tryptamine) are consistent with the phenate form being weaker than the phenolic form but the changes are smaller than with Coryneine because cinobufotenine is a weaker acid. 3 The hydroxyl group makes a large contribution to activity. Cinobufotenine is 9 times as active as the analogue without a hydroxyl on the frog rectus and 12 times as active as it on the ileum. The 5-methoxy analogue is an antagonist on the frog rectus and a very weak partial agonist on the ileum. 4 Cinobufotenine and the quaternary derivative of tryptamine have less than one-thousandth of the activity of 5-hydroxytryptamine on the rat fundus strip.
The ionization of phenolic amines, including apomorphine, dopamine and catecholamines and an assessment of zwitterion constants.[Pubmed:963339]
Br J Pharmacol. 1976 Aug;57(4):501-16.
The dissociation constants of many phenolic amines, including benzylamines, phenethylamines, phenylethanolamines, phenylpropylamines, catecholamines, and apomorphine have been measured by potentiometric titration at 25 degrees C. Measurements have also been made with many of their methoxy derivatives and with series of phenolic quaternary ammonium salts. Some compounds were also studied at 37 degrees C. 2 Usually at least five titrations were made with each compound and Debye--Huckel theory was applied to convert concentrations to activities but the estimates of pKa were not constant and found to increase with increasing concentration. The range studied was usually 5-15 mM and a least-squares line-fit, based on the empirical assumption that pKa varies with (concentration)1/2, has been used to calculate values for 10 mM solutions and to extrapolate to infinite dilution and to 100 mM. The dependence of pKa on concentration was much less at 37 degrees C than at 25 degrees C. 3 At 37 degrees C the pKa values of many biologically interesting compounds in the group, dopamine, noradrenaline, adrenaline and isoprenaline, Coryneine (the trimethylammonium derivative of dopamine) and apomorphine are within 1 log unit of physiological pH, indicating the presence of a significant proportion of either the zwitterion or of the uncharged phenolic amine. 4 Zwitterion constants have been estimated from the pKa values of the phenolic amines and those of their methoxy and quaternary trimethylammonium analogues. Zwitterion formation does not appear to be associated with activity at alpha-adrenoceptors and probably not with activity at beta-receptors. The active species seems likely to contain the unionised phenolic group but at dopamine receptors this may be in the uncharged phenolic amine rather than in the phenolic ammonium salt.
The effects of pH on the activity of coryneine and related phenolic quaternary ammonium salts on the frog rectus preparation.[Pubmed:9174]
Br J Pharmacol. 1976 Aug;57(4):517-9.
The activity of m-hydroxybenzyltrimethylammonium, Coryneine (3:4-dihydroxyphenethyltrimethylammonium, 'quaternary dopamine'), and m-hydroxyphenylpropyltrimethylammonium relative to tetramethylammonium has been measured on the frog rectus preparation (Rana pipiens) at pH 7 and pH 9. 2 The compounds are more active in the more acid environment indicating that ionization of the phenolic group reduces activity to between one-half and one-tenth of that of the form with the intact hydroxyl group. 3 In contrast with the situation at aminoacid receptors, there is no reason to believe that at other receptors zwitterions are likely to be more active than the uncharged forms with which they are in equilibrium.
The specificity of some agonists and antagonists for nicotine-sensitive receptors in ganglia.[Pubmed:4155978]
Br J Pharmacol. 1974 Aug;51(4):585-97.
1 The guinea-pig isolated ileum has been used to estimate the ability of substituted phenylalkylonium salts (related to nicotine) to stimulate or block receptors in ganglia. The effects of hexamethonium were used to indicate which were the most specific ganglion stimulants; these were tested on the blood-pressure of pithed rats and for neuromuscular blocking activity on the rat diaphragm preparation.2m-Hydroxyphenylpropyltrimethylammonium and 3,4-dihydroxyphenethyltrimethylammonium (Coryneine, ;quaternary dopamine') were the most active and specific ganglion stimulants but their usefulness in vivo may be limited by their neuromuscular blocking activity. The analogous tertiary compounds are being investigated.3 The affinities of substances which were blocking agents at ganglionic receptors were measured on the isolated ileum with m-hydroxyphenylpropyltrimethylammonium as agonist. The affinities of selected compounds for postganglionic receptors were measured in experiments on the ileum in the presence of hexamethonium and with carbachol as agonist. Some of the compounds were tested for neuromuscular blocking activity on the rat diaphragm.4 Phenylbutyldiethylamine had ganglion-blocking activity greater than pempidine and little postganglionic blocking or neuromuscular blocking activity. Its triethylammonium analogue had higher ganglion-blocking activity but had appreciable neuromuscular blocking activity.5 The aromatic ring system is not essential either for activity or affinity and the effects of substituents are not related to their effects on electron distribution. Stimulant activity is enhanced only by hydroxyl or amino groups in suitable positions; it is not improved by the presence of rigid features (double or triple bonds or a cyclopropane ring) in the side chain. Affinity is slightly increased by chloro or bromo groups in suitable positions but the unsubstituted compounds are among those with the highest affinity. Substituents have similar effects on affinity for postganglionic receptors, though for these receptors the compounds mostly have only about one-tenth of their affinity for ganglionic receptors.
Alkaloids as inhibitors of photophosphorylation in spinach chloroplasts.[Pubmed:19397001]
Biochim Biophys Acta. 1974 Jan 18;333(1):141-8.
A group of 12 alkaloids were tested as inhibitors of photophosphorylation in spinach chloroplasts. Ajmaline, a dihydroindole alkaloid, was found to be the strongest inhibitor of both cyclic and non-cyclic photophosphorylation. Low concentrations of ajmaline also inhibited the dark and light ATPases, and the coupled electron flow from water to ferricyanide, measured either as ferrocyanide formed or as oxygen evolved, but not the uncoupled electron transport or the pH rise of illuminated unbuffered suspensions of chloroplasts. Higher concentrations of ajmaline stimulated, instead of inhibiting, photosynthetic electron transport or oxygen evolution and decreased the pH rise, thus behaving as an uncoupler, such as ammonia. Photophosphorylation was partially inhibited by 100 microM dihydrosanguinarine, 100 microM dihydrochelerythrine (benzophenanthridine alkaloids); 500 microM O,O'-dimethylmagnoflorine, 500 microM N-methylcorydine (aporphine alkaloids) and 1 mM julocrotine. They also inhibited coupled oxygen evolution and only partially (dihydrosanguinarine and dihydrochelerythrine) or not at all (the other alkaloids) uncoupled oxygen evolution. Spegazzinine (dihydroindole alkaloid), magnoflorine, N-methylisocorydine, Coryneine (aporphine alkaloids), candicine and ribalinium chloride were without effect on photophosphorylation at 500 microM.


