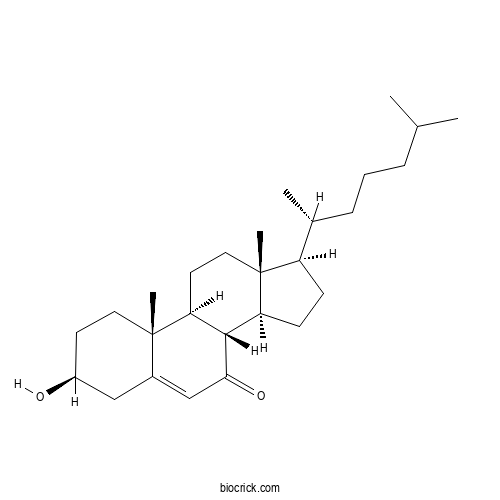
7-KetocholesterolCAS# 566-28-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 566-28-9 | SDF | Download SDF |
| PubChem ID | 91474 | Appearance | Powder |
| Formula | C27H44O2 | M.Wt | 400.6 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,8S,9S,10R,13R,14S,17R)-3-hydroxy-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-1,2,3,4,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-7-one | ||
| SMILES | CC(C)CCCC(C)C1CCC2C1(CCC3C2C(=O)C=C4C3(CCC(C4)O)C)C | ||
| Standard InChIKey | YIKKMWSQVKJCOP-ABXCMAEBSA-N | ||
| Standard InChI | InChI=1S/C27H44O2/c1-17(2)7-6-8-18(3)21-9-10-22-25-23(12-14-27(21,22)5)26(4)13-11-20(28)15-19(26)16-24(25)29/h16-18,20-23,25,28H,6-15H2,1-5H3/t18-,20+,21-,22+,23+,25+,26+,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

7-Ketocholesterol Dilution Calculator

7-Ketocholesterol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4963 mL | 12.4813 mL | 24.9626 mL | 49.9251 mL | 62.4064 mL |
| 5 mM | 0.4993 mL | 2.4963 mL | 4.9925 mL | 9.985 mL | 12.4813 mL |
| 10 mM | 0.2496 mL | 1.2481 mL | 2.4963 mL | 4.9925 mL | 6.2406 mL |
| 50 mM | 0.0499 mL | 0.2496 mL | 0.4993 mL | 0.9985 mL | 1.2481 mL |
| 100 mM | 0.025 mL | 0.1248 mL | 0.2496 mL | 0.4993 mL | 0.6241 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4',5-Di-O-methyl quercetin
Catalog No.:BCN0211
CAS No.:100648-56-4
- Lathyranoic acid A
Catalog No.:BCN0210
CAS No.:850560-44-0
- Isophysalin G
Catalog No.:BCN0209
CAS No.:152221-21-1
- (3beta,22alpha)-26-(beta-glucopyranosyloxy)-22-methoxyfurost-5-en-3-yl 2-O-(6-deoxy-alpha-mannopyranosyl)-beta-glucopyranosiduronic acid
Catalog No.:BCN0208
CAS No.:107783-53-9
- 9,11-Dehydro-beta-boswellic acid
Catalog No.:BCN0207
CAS No.:471-65-8
- Isocucurbitacin D
Catalog No.:BCN0206
CAS No.:68422-20-8
- Cuscutamine
Catalog No.:BCN0205
CAS No.:122170-93-8
- Batatasin IV
Catalog No.:BCN0204
CAS No.:60347-67-3
- Euphorbia factor L24
Catalog No.:BCN0203
CAS No.:1613700-13-2
- Notoginsenoside L13
Catalog No.:BCN0202
CAS No.:2485859-56-9
- Physalin X
Catalog No.:BCN0201
CAS No.:72497-31-5
- Arvenin III
Catalog No.:BCN0200
CAS No.:65597-45-7
- 3-Oxo-olean-12-en-28-oic acid methyl ester
Catalog No.:BCN0213
CAS No.:1721-58-0
- Physalin F
Catalog No.:BCN0214
CAS No.:57423-71-9
- 4-Methyl-6-phenyl-2H-pyranone
Catalog No.:BCN0215
CAS No.:4467-30-5
- Rutarensin
Catalog No.:BCN0216
CAS No.:119179-04-3
- 3,5,6,7,8,4'-hexamethoxyflavone
Catalog No.:BCN0217
CAS No.:34170-18-8
- 3',5-Di-O-methyl quercetin
Catalog No.:BCN0218
CAS No.:40554-94-7
- Coryneine
Catalog No.:BCN0219
CAS No.:7224-66-0
- Corysamine chloride
Catalog No.:BCN0220
CAS No.:11028-77-6
- Tubuloside B
Catalog No.:BCN0221
CAS No.:112516-04-8
- Linaroside
Catalog No.:BCN0222
CAS No.:53452-12-3
- 6-O-Methylcatalpol
Catalog No.:BCN0223
CAS No.:1617-84-1
- Ricinine
Catalog No.:BCN0224
CAS No.:524-40-3
Influential role of 7- Ketocholesterol in the progression of Alzheimer's disease.[Pubmed:34273491]
Prostaglandins Other Lipid Mediat. 2021 Jul 14:106582.
Millions of people are affected by neurodegenerative diseases worldwide. It occurs due to the loss of brain functions or peripheral nervous system dysfunction. If untreated, prolonged condition ultimately it leads to death. Mostly it is associated with stress, altered cholesterol metabolism, in fl ammation and organelle dysfunction. Endogenous cholesterol and phospholipids in brain undergo auto-oxidation by enzymatic as well as non-enzymatic modes leading to the formation of by-products such as 4-hydroxynonenal and oxysterols. Among various oxysterols, 7-Ketocholesterol (7KCh) is one of the major toxic components involved in altering neuronal lipid metabolism, contributing to in fl ammation and nerve cell damage. More evidently 7KCh is proven to induce oxidative stress and affects membrane permeability. Loss in mitochondrial membrane potential affects metabolism of cell organelles such as lysosomes and peroxisomes which are involved in lipid and protein homeostasis. This in turn could affect amyloidogenesis, tau protein phosphorylation and accumulation in pathological conditions of neurodegenerative diseases. Lipid alterations and the consequent pathogenic protein accumulation, results in the damage of cell organelles and microglial cells. This could be a reason behind disease progression and predominantly reported characteristics of neurodegenerative disorders such as Alzheimer's disease. This review focuses on the role of 7KCh mediated neurodegenerative Alzheimer's disease with emphasis on alterations in the lipid raft microdomain. In addition, current trends in the significant therapies related to 7KCh inhibition are highlighted.
Potential Disease-Modifying Effects of Lithium Carbonate in Niemann-Pick Disease, Type C1.[Pubmed:34177581]
Front Pharmacol. 2021 Jun 9;12:667361.
Background: Niemann-Pick disease type C1 (NP-C1) is a rare, autosomal-recessive neurodegenerative disorder with no United States Food and Drug Administration (FDA)-approved drug. Lithium has been shown to have considerable neuroprotective effects for neurological disorders such as bipolar disorder, Alzheimer's disease and stroke and has been tested in many clinical trials. However, the pharmacological effect of lithium on NP-C1 neurodegenerative processes has not been investigated. The aim of this study was to provide an initial evaluation of the safety and feasibility of lithium carbonate in patients with NP-C1. Methods: A total of 13 patients diagnosed with NP-C1 who met the inclusion criteria received lithium orally at doses of 300, 600, 900, or 1,200 mg daily. The dose was reduced based on tolerance or safety observations. Plasma 7-Ketocholesterol (7-KC), an emerging biomarker of NP-C1, was the primary endpoint. Secondary endpoints included NPC Neurological Severity Scores (NNSS) and safety. Results: Of the 13 patients with NP-C1 (12-33 years) enrolled, three withdrew (discontinuation of follow-up outpatient visits). The last observed post-treatment values of 7-KC concentrations (128 ng/ml, SEM 20) were significantly lower than pretreatment baselines values (185 ng/ml, SEM 29; p = 0.001). The mean NNSS was improved after lithium treatment at 12 months (p = 0.005). Improvement in swallowing capacity was observed in treated patients (p = 0.014). No serious adverse events were recorded in the patients receiving lithium. Conclusion: Lithium is a potential therapeutic option for NP-C1 patients. Larger randomized and double-blind clinical trials are needed to further support this finding. Clinical Trial Registration: ClinicalTrials.gov, NCT03201627.
7-Ketocholesterol: Effects on viral infections and hypothetical contribution in COVID-19.[Pubmed:34118414]
J Steroid Biochem Mol Biol. 2021 Jun 9;212:105939.
7-Ketocholesterol, which is one of the earliest cholesterol oxidization products identified, is essentially formed by the auto-oxidation of cholesterol. In the body, 7-Ketocholesterol is both provided by food and produced endogenously. This pro-oxidant and pro-inflammatory molecule, which can activate apoptosis and autophagy at high concentrations, is an abundant component of oxidized Low Density Lipoproteins. 7-Ketocholesterol appears to significantly contribute to the development of age-related diseases (cardiovascular diseases, age-related macular degeneration, and Alzheimer's disease), chronic inflammatory bowel diseases and to certain cancers. Recent studies have also shown that 7-Ketocholesterol has anti-viral activities, including on SARS-CoV-2, which are, however, lower than those of oxysterols resulting from the oxidation of cholesterol on the side chain. Furthermore, 7-Ketocholesterol is increased in the serum of moderately and severely affected COVID-19 patients. In the case of COVID-19, it can be assumed that the antiviral activity of 7-Ketocholesterol could be counterbalanced by its toxic effects, including pro-oxidant, pro-inflammatory and pro-coagulant activities that might promote the induction of cell death in alveolar cells. It is therefore suggested that this oxysterol might be involved in the pathophysiology of COVID-19 by contributing to the acute respiratory distress syndrome and promoting a deleterious, even fatal outcome. Thus, 7-Ketocholesterol could possibly constitute a lipid biomarker of COVID-19 outcome and counteracting its toxic effects with adjuvant therapies might have beneficial effects in COVID-19 patients.
The Controversial Role of 24-S-Hydroxycholesterol in Alzheimer's Disease.[Pubmed:34067119]
Antioxidants (Basel). 2021 May 7;10(5). pii: antiox10050740.
The development of Alzheimer's disease (AD) is influenced by several events, among which the dysregulation of cholesterol metabolism in the brain plays a major role. Maintenance of brain cholesterol homeostasis is essential for neuronal functioning and brain development. To maintain the steady-state level, excess brain cholesterol is converted into the more hydrophilic metabolite 24-S-hydroxycholesterol (24-OHC), also called cerebrosterol, by the neuron-specific enzyme CYP46A1. A growing bulk of evidence suggests that cholesterol oxidation products, named oxysterols, are the link connecting altered cholesterol metabolism to AD. It has been shown that the levels of some oxysterols, including 27-hydroxycholesterol, 7beta-hydroxycholesterol and 7-Ketocholesterol, significantly increase in AD brains contributing to disease progression. In contrast, 24-OHC levels decrease, likely due to neuronal loss. Among the different brain oxysterols, 24-OHC is certainly the one whose role is most controversial. It is the dominant oxysterol in the brain and evidence shows that it represents a signaling molecule of great importance for brain function. However, numerous studies highlighted the potential role of 24-OHC in favoring AD development, since it promotes neuroinflammation, amyloid beta (Abeta) peptide production, oxidative stress and cell death. In parallel, 24-OHC has been shown to exert several beneficial effects against AD progression, such as preventing tau hyperphosphorylation and Abeta production. In this review we focus on the current knowledge of the controversial role of 24-OHC in AD pathogenesis, reporting a detailed overview of the findings about its levels in different AD biological samples and its noxious or neuroprotective effects in the brain. Given the relevant role of 24-OHC in AD pathophysiology, its targeting could be useful for disease prevention or slowing down its progression.
7-ketocholesterol enhances autophagy via the ROS-TFEB signaling pathway in osteoclasts.[Pubmed:34023424]
J Nutr Biochem. 2021 May 21;96:108783.
Oxysterols play a critical role in human health and diseases associated with high cholesterol and oxidative stress. Given that a positive correlation was observed between cholesterol and collagen type 1 fragment (CTX-1) or serum reactive oxygen species (ROS) in humans, we hypothesized that oxidized cholesterol metabolites may participate in cholesterol-induced bone loss. Therefore, this study aimed to identify the metabolite responsible for cholesterol-associated bone loss and evaluate its effect on osteoclasts (OCs) leading to bone loss. An atherogenic diet in mice increased the levels of the oxysterol, 7-Ketocholesterol (7-KC) in bone, as well as serum ROS. 7-KC increased the number and activity of OCs by enhancing autophagy via the ROS-transcription factor EB signaling pathway. These findings suggest that 7-KC acts as a cholesterol metabolite and is at least partially responsible for cholesterol-induced bone loss by inducing autophagy in OCs.
An insight on 7- ketocholesterol mediated inflammation in atherosclerosis and potential therapeutics.[Pubmed:33930389]
Steroids. 2021 Aug;172:108854.
7-Ketocholesterol, a toxic oxidative product of oxysterol is a causative agent of several diseases and disabilities concomitant to aging including cardiovascular diseases like atherosclerosis. Auto-oxidation of cholesterol esters present in low-density lipoprotein (LDL) deposits lead to the formation of oxidized LDL (Ox-LDL) along with its byproducts, namely 7KCh. It is predominantly found in atherosclerotic plaque and also found to be more atherogenic than cholesterol by being cytotoxic, interfering with cellular homeostasis. This makes it a serious threat by being the foremost cause of morbidity and mortality worldwide and is likely to become more serious during forth coming years. It involves in mediating inflammatory mechanisms characterized by the advancement of fibroatheroma plaques. The atherosclerotic lesion is composed of Ox-LDL along with fibrotic mass consisting of immune cells and molecules. Macrophages being the specialized phagocytic cells, contribute to removal of detrimental contents of the lesion along with accumulated lipids leading to alteration of its biology and functionality due to its plasticity. Here, we have explored the known as well as proposed mechanisms involved with 7KCh associated atherogenesis along with potential therapeutic strategies for targeting 7KCh as a diagnostic and target in medicine.
Oxysterol species generated by auto-oxidation in subclinical hypothyroidism.[Pubmed:33861988]
Clin Biochem. 2021 Jul;93:73-79.
BACKGROUND: Auto-oxidized oxysterols are implicated in the pathogenesis of various chronic diseases. Their concentrations are indicators of oxidative stress in vivo and associated with atherosclerosis. Subclinical hypothyroidism is related with cardiac diseases and oxidative stress, but the exact mechanisms underlying these associations are not clear yet. OBJECTIVE: To investigate the auto-oxidized oxysterols, 7-Ketocholesterol (7-KC) and cholestane-3beta,5alpha,6beta-triol (chol-triol), in patients with subclinical hypothyroidism, as well as to evaluate the impact of restoring euthyroidism on oxysterol concentrations. METHODS: In this prospective observational study, 64 patients with newly diagnosed autoimmune thyroiditis (41 with subclinical hypothyroidism and 23 euthyroidism), and 45 healthy controls were enrolled. Age, gender, and body mass index were matched among patient groups and healthy controls. Anthropometric measurements were obtained and fasting plasma 7-Ketocholesterol and cholestane-3beta,5alpha,6beta-triol concentrations were measured by using liquid chromatography coupled with tandem mass spectrometry. Levothyroxine was then administered to all patients with subclinical-hypothyroidism. After three months, measurements of the oxysterols and serum cholesterols from the patients who have become euthyroid were repeated. RESULTS: Concentrations of 7-Ketocholesterol and cholestane-3beta,5alpha,6beta-triol were significantly higher in patients with subclinical-hypothyroidism when compared to both euthyroid patients and healthy controls (p < 0.001 for both oxysterols). After restoration of euthyroidism, concentrations of 7-Ketocholesterol and cholestane-3beta,5alpha,6beta-triol decreased significantly and reached similar concentrations observed in healthy controls (p < 0.001 for both oxysterols). CONCLUSIONS: Auto-oxidized oxysterol species are higher in patients with mild thyroid dysfunction, and supported the rationale for treating subclinical-hypothyroidism.
Cyclodextrin dimers: a versatile approach to optimizing encapsulation and their application to therapeutic extraction of toxic oxysterols.[Pubmed:33839224]
Int J Pharm. 2021 Apr 8:120522.
We have developed a novel class of specifically engineered, dimerized cyclodextrin nanostructures for the encapsulation of toxic biomolecules such as 7-Ketocholesterol (7KC). 7KC accumulates over time and causes dysfunction in many cell types, linking it to several age-related diseases including atherosclerosis and age-related macular degeneration (AMD). Presently, treatments for these diseases are invasive, expensive, and show limited benefits. Cyclodextrins (CDs) are cyclic glucose oligomers utilized to capture small, hydrophobic molecules. Here, a combination of in silico, in vitro, and ex vivo methods is used to implement a synergistic rational drug design strategy for developing CDs to remove atherogenic 7KC from cells and tissues. Mechanisms by which CDs encapsulate sterols are discussed, and we conclude that covalently linked head-to-head dimers of betaCDs have substantially improved affinity for 7KC compared to monomers. We find that inclusion complexes can be stabilized or destabilized in ways that allow the design of CD dimers with increased 7KC selectivity while maintaining an excellent safety profile. These CD dimers are being developed as therapeutics to treat atherosclerosis and other debilitating diseases of aging.
Identification of Anti-Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Oxysterol Derivatives In Vitro.[Pubmed:33808940]
Int J Mol Sci. 2021 Mar 19;22(6). pii: ijms22063163.
The development of effective antiviral drugs targeting the severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) is urgently needed to combat the coronavirus disease 2019 (COVID-19). We have previously studied the use of semi-synthetic derivatives of oxysterols, oxidized derivatives of cholesterol as drug candidates for the inhibition of cancer, fibrosis, and bone regeneration. In this study, we screened a panel of naturally occurring and semi-synthetic oxysterols for anti-SARS-CoV-2 activity using a cell culture infection assay. We show that the natural oxysterols, 7-Ketocholesterol, 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol, and 27-hydroxycholesterol, substantially inhibited SARS-CoV-2 propagation in cultured cells. Among semi-synthetic oxysterols, Oxy210 and Oxy232 displayed more robust anti-SARS-CoV-2 activities, reducing viral replication more than 90% at 10 muM and 99% at 15 muM, respectively. When orally administered in mice, peak plasma concentrations of Oxy210 fell into a therapeutically relevant range (19 muM), based on the dose-dependent curve for antiviral activity in our cell-based assay. Mechanistic studies suggest that Oxy210 reduced replication of SARS-CoV-2 by disrupting the formation of double-membrane vesicles (DMVs); intracellular membrane compartments associated with viral replication. Our study warrants further evaluation of Oxy210 and Oxy232 as a safe and reliable oral medication, which could help protect vulnerable populations with increased risk of developing COVID-19.
Dietary Oxysterol, 7-Ketocholesterol Accelerates Hepatic Lipid Accumulation and Macrophage Infiltration in Obese Mice.[Pubmed:33776901]
Front Endocrinol (Lausanne). 2021 Mar 10;11:614692.
Non-alcoholic fatty liver disease is strongly associated with obese and type 2 diabetes. It has been reported that an oxidized cholesterol, 7-Ketocholesterol (7KC), might cause inflammatory response in macrophages and plasma 7KC concentration were higher in patients with cardiovascular diseases or diabetes. Therefore, we have decided to test whether small amount of 7KC in diet might induce hepatic steatosis and inflammation in two types of obese models. We found that addition of 0.01% 7KC either in chow diet (CD, regular chow diet with 1% cholesterol) or western type diet (WD, high fat diet with 1% cholesterol) accelerated hepatic neutral lipid accumulation by Oil Red O staining. Importantly, by lipid extraction analysis, it has been recognized that triglyceride rather than cholesterol species was significantly accumulated in CD+7KC compared to CD as well as in WD+7KC compared to WD. Immunostaining revealed that macrophages infiltration was increased in CD+7KC compared to CD, and also in WD+7KC compared to WD. These phenotypes were accompanied by inducing inflammatory response and downregulating fatty acid oxidation. Furthermore, RNA sequence analysis demonstrated that 7KC reduced expression of genes which related to autophagy process. Levels of LC3-II protein were decreased in WD+7KC compared to WD. Similarly, we have confirmed the effect of 7KC on acceleration of steatohepatitis in db/db mice model. Collectively, our study has demonstrated that small amount of dietary 7KC contributed to accelerate hepatic steatosis and inflammation in obese mice models.
Attenuation of 7-ketocholesterol- and 7beta-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: Potential for the prevention of age-related diseases.[Pubmed:33774195]
Ageing Res Rev. 2021 Jul;68:101324.
Age-related diseases for which there are no effective treatments include cardiovascular diseases; neurodegenerative diseases such as Alzheimer's disease; eye disorders such as cataract and age-related macular degeneration; and, more recently, Severe Acute Respiratory Syndrome (SARS-CoV-2). These diseases are associated with plasma and/or tissue increases in cholesterol derivatives mainly formed by auto-oxidation: 7-Ketocholesterol, also known as 7-oxo-cholesterol, and 7beta-hydroxycholesterol. The formation of these oxysterols can be considered as a consequence of mitochondrial and peroxisomal dysfunction, leading to increased in oxidative stress, which is accentuated with age. 7-Ketocholesterol and 7beta-hydroxycholesterol cause a specific form of cytotoxic activity defined as oxiapoptophagy, including oxidative stress and induction of death by apoptosis associated with autophagic criteria. Oxiaptophagy is associated with organelle dysfunction and in particular with mitochondrial and peroxisomal alterations involved in the induction of cell death and in the rupture of redox balance. As the criteria characterizing 7-Ketocholesterol- and 7beta-hydroxycholesterol-induced cytotoxicity are often simultaneously observed in major age-related diseases (cardiovascular diseases, age-related macular degeneration, Alzheimer's disease) the involvement of these oxysterols in the pathophysiology of the latter seems increasingly likely. It is therefore important to better understand the signalling pathways associated with the toxicity of 7-Ketocholesterol and 7beta-hydroxycholesterol in order to identify pharmacological targets, nutrients and synthetic molecules attenuating or inhibiting the cytotoxic activities of these oxysterols. Numerous natural cytoprotective compounds have been identified: vitamins, fatty acids, polyphenols, terpenes, vegetal pigments, antioxidants, mixtures of compounds (oils, plant extracts) and bacterial enzymes. However, few synthetic molecules are able to prevent 7-Ketocholesterol- and/or 7beta-hydroxycholesterol-induced cytotoxicity: dimethyl fumarate, monomethyl fumarate, the tyrosine kinase inhibitor AG126, memantine, simvastatine, Trolox, dimethylsufoxide, mangafodipir and mitochondrial permeability transition pore (MPTP) inhibitors. The effectiveness of these compounds, several of which are already in use in humans, makes it possible to consider using them for the treatment of certain age-related diseases associated with increased plasma and/or tissue levels of 7-Ketocholesterol and/or 7beta-hydroxycholesterol.
Lipid profiling and dietary assessment of infant formulas reveal high intakes of major cholesterol oxidative product (7-ketocholesterol).[Pubmed:33761334]
Food Chem. 2021 Aug 30;354:129529.
Approximately two-thirds of US infants receive infant formula (IF) as a primary or sole nutritional source during the first six months of life. IF is available in a variety of commercial presentations; from a manufacturing standpoint, they can be categorized as powder- (PIF) or liquid- (LIF) based formulations. Thirty commercial IFs were analyzed in their oxidative and non-oxidative lipid profiles. We identified 7-Ketocholesterol - a major end-product of cholesterol oxidation - as a potential biomarker of IF manufacturing. The statistical analysis allowed a re-classification of IF based on their metabolomic fingerprint, resulting in three groups assigned with low-to-high oxidative status. Finally, we modeled the dietary intake of cholesterol, sterols, and 7-Ketocholesterol in the first year of life. The database provided in this study will be instrumental for scientists interested in infant nutrition, to establish bases for epidemiological studies aimed to find connections between nutrition and diet-associated diseases, such as sitosterolemia.
Fatty Acid Composition and Stoichiometry Determine the Angiogenesis Microenvironment.[Pubmed:33681633]
ACS Omega. 2021 Feb 16;6(8):5953-5961.
The current study tested the hypothesis of whether specific lipids may control angiogenic reactions. Using the chorioallantoic membrane assay of the chick embryo, new vessel formation was analyzed quantitatively by gas chromatography and mass spectrometry as well as bioinformatics tools including an angiogenesis analyzer. Our biochemical experiments showed that a specific lipid composition and stoichiometry determine the angiogenesis microenvironment to accelerate or inhibit vessel formation. Specific lipids of angiogenesis determinants in the vessel area and the non-vessel area were identified as nitrooleic acid, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), palmitic acid, oleic acid, linoleic acid, linolenic acid, epoxyoleic acid, lysophosphatidylcholine (LPC), cholesterol, 7-Ketocholesterol, and docosahexaenoyl lysophosphatidylcholine (DHA-LPC). Vessel formation happens on the surface area of the hydrophilic membrane of the yolk. Our biochemical data demonstrated that angiogenesis was followed in the white lipid complex area to generate more branches, junctions, segments, and extremities. We analyzed lipid fragments in the vessel, non-vessel, and albumen area to show that each area contains a specific lipid composition and stoichiometry. Mass spectrometry data demonstrated that the vessel area has higher concentrations of nitrooleic acid, palmitic acid, stearic acid, LPC, lysophosphatidylethanolamine, cholesterol, oleic acid, linoleic acid, 7-Ketocholesterol, and DHA-LPC; however, DHA and EPA were abundant in the hydrophobic non-vessel area. The purpose of vessel formation is to wrap up the yolk area to transport nutrients including specific fatty acids. Besides, angiogenesis requires aqueous albumen shown by distance-dependent vessel formation from albumen and oxygen. Higher concentrations of fatty acids are required for energy and carbon structure from the carbon-carbon bond, membrane building blocks, and amphiphilic detergent to solubilize a hydrophobic environment in the aqueous blood layer. The current study may guide that the uncovered hydrophobic or zwitterionic molecules such as DHA and DHA-LPC may control angiogenesis as antiangiogenic or proangiogenic molecules as potential drug targets for treating uncontrolled angiogenesis-related diseases, including diabetic retinopathy and age-related macular degeneration.
The cholesterol autoxidation products, 7-ketocholesterol and 7beta-hydroxycholesterol are associated with serum neurofilaments in multiple sclerosis.[Pubmed:33677412]
Mult Scler Relat Disord. 2021 May;50:102864.
BACKGROUND: Serum neurofilament light chain (sNfL) is an established marker of neuroaxonal injury in multiple sclerosis (MS). OBJECTIVES: To investigate if oxysterols produced from non-enzymatic and enzymatic cholesterol oxidation are differentially associated with sNfL measurements in MS. METHODS: This longitudinal study included 62 relapsing-remitting (RR-MS) and 36 progressive MS (PMS) patients with baseline and 5-year follow-up measures of serum levels of 6 oxysterols, sNfL and lipids. The oxysterols, 24-hydroxycholesterol (24HC), 25HC, 27HC, 7alphaHC, 7betaHC and 7-Ketocholesterol (7KC), were measured using liquid chromatography-mass spectrometry. sNfL was measured using single molecular array assay. Serum high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) levels were obtained from a lipid profile. RESULTS: The enzymatically produced oxysterols 24HC, 25HC, 27HC and 7alphaHC were not associated with sNfL. However, baseline levels of reactive oxygen species (ROS) produced oxysterols, 7KC (p = 0.032) and 7betaHC (p = 0.0025), were positively associated with sNfL levels at follow-up. Follow-up 7KC (p = 0.038) levels were also associated with follow-up sNfL levels. The associations of 7KC or 7betaHC with sNfL remained significant after adjusting for LDL-C or HDL-C. CONCLUSIONS: 7KC and 7betaHC, produced by ROS-mediated cholesterol oxidation are associated with neuroaxonal injury as assessed by sNfL in MS.
Clinical, biochemical, and genotype-phenotype correlations of 118 patients with Niemann-Pick disease Types A/B.[Pubmed:33675270]
Hum Mutat. 2021 May;42(5):614-625.
Niemann-Pick disease Types A and B (NPA/B) are autosomal recessive disorders caused by variants in the sphingomyelin phosphodiesterase-1 (SMPD1) gene. This study aimed to describe and characterize a cohort of 118 patients diagnosed with NPA/B based on clinical, biochemical, and molecular findings, and to identify sound correlations between laboratory findings and clinical presentations. Decreased peripheral leukocyte acid sphingomyelinase activity levels and increased plasma 7-Ketocholesterol levels were significantly correlated with disease onset and severity of the clinical course. We identified 92 different sequence SMPD1 variants, including 41 novel variants, in 118 NPA/B patients (19 NPA, 24 intermediate type, 75 NPB). The most prevalent mutation was p.Arg602His, which accounted for 9.3% of the alleles. Patients homozygous for p.Arg602His or p.Asn522Ser showed a late-onset form of the NPB phenotype. The homozygous SMPD1 variant p.Tyr500His correlated with the early-onset NPB clinical form. Additionally, homozygous variants p.His284SerfsX18, p.Phe465Ser, and p.Ser486Arg were associated with the neuronopathic NPA clinical form. The homozygous variant p.Arg3AlafsX74 was associated with the intermediate clinical form. Our study contributes to the understanding of the natural history of NPA/B and assists in the development of efficacious treatments for patients afflicted with this devastating lysosomal storage disorder.


