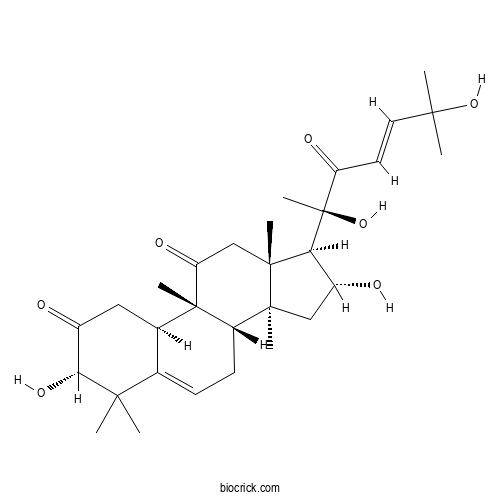
Isocucurbitacin DCAS# 68422-20-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68422-20-8 | SDF | Download SDF |
| PubChem ID | 6325422 | Appearance | Powder |
| Formula | C30H44O7 | M.Wt | 516.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,8S,9R,10R,13R,14S,16R,17R)-17-[(E,2R)-2,6-dihydroxy-6-methyl-3-oxohept-4-en-2-yl]-3,16-dihydroxy-4,4,9,13,14-pentamethyl-3,7,8,10,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthrene-2,11-dione | ||
| SMILES | CC1(C(C(=O)CC2C1=CCC3C2(C(=O)CC4(C3(CC(C4C(C)(C(=O)C=CC(C)(C)O)O)O)C)C)C)O)C | ||
| Standard InChIKey | ALPSEMFPAVSKJO-DJMAWCNKSA-N | ||
| Standard InChI | InChI=1S/C30H44O7/c1-25(2,36)12-11-21(33)30(8,37)23-19(32)14-27(5)20-10-9-16-17(13-18(31)24(35)26(16,3)4)29(20,7)22(34)15-28(23,27)6/h9,11-12,17,19-20,23-24,32,35-37H,10,13-15H2,1-8H3/b12-11+/t17-,19-,20+,23+,24-,27+,28-,29+,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isocucurbitacin D Dilution Calculator

Isocucurbitacin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9354 mL | 9.6768 mL | 19.3536 mL | 38.7072 mL | 48.384 mL |
| 5 mM | 0.3871 mL | 1.9354 mL | 3.8707 mL | 7.7414 mL | 9.6768 mL |
| 10 mM | 0.1935 mL | 0.9677 mL | 1.9354 mL | 3.8707 mL | 4.8384 mL |
| 50 mM | 0.0387 mL | 0.1935 mL | 0.3871 mL | 0.7741 mL | 0.9677 mL |
| 100 mM | 0.0194 mL | 0.0968 mL | 0.1935 mL | 0.3871 mL | 0.4838 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cuscutamine
Catalog No.:BCN0205
CAS No.:122170-93-8
- Batatasin IV
Catalog No.:BCN0204
CAS No.:60347-67-3
- Euphorbia factor L24
Catalog No.:BCN0203
CAS No.:1613700-13-2
- Notoginsenoside L13
Catalog No.:BCN0202
CAS No.:2485859-56-9
- Physalin X
Catalog No.:BCN0201
CAS No.:72497-31-5
- Arvenin III
Catalog No.:BCN0200
CAS No.:65597-45-7
- Parishin G
Catalog No.:BCN0199
CAS No.:952283-93-1
- alpha-Costic acid
Catalog No.:BCN0198
CAS No.:28399-17-9
- Batatasin V
Catalog No.:BCN0197
CAS No.:65817-45-0
- Menisperine
Catalog No.:BCN0196
CAS No.:25342-82-9
- 8'-O-(3-hydroxy-3-methylglutaryl)-8'-hydroxyabscisic acid
Catalog No.:BCN0195
CAS No.:69790-31-4
- Isovitexin-2''-O-rhamnoside
Catalog No.:BCN0194
CAS No.:72036-50-1
- 9,11-Dehydro-beta-boswellic acid
Catalog No.:BCN0207
CAS No.:471-65-8
- (3beta,22alpha)-26-(beta-glucopyranosyloxy)-22-methoxyfurost-5-en-3-yl 2-O-(6-deoxy-alpha-mannopyranosyl)-beta-glucopyranosiduronic acid
Catalog No.:BCN0208
CAS No.:107783-53-9
- Isophysalin G
Catalog No.:BCN0209
CAS No.:152221-21-1
- Lathyranoic acid A
Catalog No.:BCN0210
CAS No.:850560-44-0
- 4',5-Di-O-methyl quercetin
Catalog No.:BCN0211
CAS No.:100648-56-4
- 7-Ketocholesterol
Catalog No.:BCN0212
CAS No.:566-28-9
- 3-Oxo-olean-12-en-28-oic acid methyl ester
Catalog No.:BCN0213
CAS No.:1721-58-0
- Physalin F
Catalog No.:BCN0214
CAS No.:57423-71-9
- 4-Methyl-6-phenyl-2H-pyranone
Catalog No.:BCN0215
CAS No.:4467-30-5
- Rutarensin
Catalog No.:BCN0216
CAS No.:119179-04-3
- 3,5,6,7,8,4'-hexamethoxyflavone
Catalog No.:BCN0217
CAS No.:34170-18-8
- 3',5-Di-O-methyl quercetin
Catalog No.:BCN0218
CAS No.:40554-94-7
Lipid-Lowering Activities of Cucurbitacins Isolated from Trichosanthes cucumeroides and Their Synthetic Derivatives.[Pubmed:33269591]
J Nat Prod. 2020 Dec 24;83(12):3536-3544.
In the ongoing efforts to discover natural cholesterol-lowering compounds, dihydrocucurbitacin B, isolated from Trichosanthes cucumeroides roots, was found to promote LDL uptake by upregulating LDLR protein in a PCSK9-dependent process. In this study, an in-depth investigation of T. cucumeroides roots afforded 27 cucurbitacins (1-27), including seven new cucurbitacins (1-7), and their structures were elucidated by spectroscopic data analyses. In order to gain insight into their structure-activity relationship, cucurbitacin derivatives (B1-11 and DB1-11) were synthesized. Evaluation of lipid-lowering activities of these cucurbitacins by an LDL uptake assay in HepG2 cells revealed that most of the compounds improved the LDL uptake rate, among which hexanorIsocucurbitacin D (6) and Isocucurbitacin D (21) exhibited the highest activities (rates of 2.53 and 2.47, respectively), which were comparable to that of the positive control, nagilactone B (rate of 2.07). According to a mechanistic study by Western blot analysis, compounds 6 and 21 dose-dependently increased LDLR protein levels and reduced PCSK9 protein levels, representing promising new lipid-lowering drug candidates.
Cucurbitacin D Is a Disruptor of the HSP90 Chaperone Machinery.[Pubmed:25756299]
J Nat Prod. 2015 Apr 24;78(4):873-9.
Heat shock protein 90 (Hsp90) facilitates the maturation of many newly synthesized and unfolded proteins (clients) via the Hsp90 chaperone cycle, in which Hsp90 forms a heteroprotein complex and relies upon cochaperones, immunophilins, etc., for assistance in client folding. Hsp90 inhibition has emerged as a strategy for anticancer therapies due to the involvement of clients in many oncogenic pathways. Inhibition of chaperone function results in client ubiquitinylation and degradation via the proteasome, ultimately leading to tumor digression. Small molecule inhibitors perturb ATPase activity at the N-terminus and include derivatives of the natural product geldanamycin. However, N-terminal inhibition also leads to induction of the pro-survival heat shock response (HSR), in which displacement of the Hsp90-bound transcription factor, heat shock factor-1, translocates to the nucleus and induces transcription of heat shock proteins, including Hsp90. An alternative strategy for Hsp90 inhibition is disruption of the Hsp90 heteroprotein complex. Disruption of the Hsp90 heteroprotein complex is an effective strategy to prevent client maturation without induction of the HSR. Cucurbitacin D, isolated from Cucurbita texana, and 3-epi-Isocucurbitacin D prevented client maturation without induction of the HSR. Cucurbitacin D also disrupted interactions between Hsp90 and two cochaperones, Cdc37 and p23.
[Studies on chemical constituents in roots of Helicteres angustifolia].[Pubmed:21842648]
Zhongguo Zhong Yao Za Zhi. 2011 May;36(9):1193-7.
OBJECTIVE: To study the chemical constituents of the roots of Helicteres angustifolia. METHOD: The compounds were isolated and purified by column chromatographic methods on silica gel, Sephadex LH-20, ODS and preparative HPLC. Their structures were elucidated on the basis of physicochemical properties and spectral data. RESULTS: Fourteen compounds were isolated from this plant. Their structures were identified as methyl helicterate(1),3-acetoxybetulin(2),3beta-acetoxy-27-(p-hydroxyl)benzoyloxylup-20( 29)-en-28-oic acid methyl ester(3),3beta-acetoxy-27-benzoyloxylup-20(29)-en-28-oic acid(4),3beta-acetoxybetulinic acid(5),pyracrenic acid(6),cucurbitacin D(7),cucurbitacin B(8),Isocucurbitacin D(9),3beta-acetoxy-27-[(4-hydroxybenzoyl)oxy]olean-12-en-28-oic acid methyl ester (10),beta-sitosterol(11),2alpha,7beta,20alpha-trihydroxy-3beta,21-dimethoxy-5-pre gnene(12), hexadecanoic acid(13), and daucosterol(14), respectively. CONCLUSION: Compounds 5,8,9,13, 14 were isolated from this plant for the first time.
A new cucurbitacin from Bolbostemma paniculatum Franguent.[Pubmed:17454317]
J Asian Nat Prod Res. 2007 Mar;9(2):187-90.
A new cucurbitacin with an unusual ring A, Isocucurbitacin D 25-O-acetate (1), was isolated from Bolbostemma paniculatum Franguent together with one known compound, cucurbitacin E (2). The structure of new compound was established by spectroscopic methods.
Cucurbitacins fromCucurbita texana: Evidence for the role of isocucurbitacins.[Pubmed:24248508]
J Chem Ecol. 1993 Jan;19(1):29-37.
In addition to cucurbitacins E andI, cucurbitacins D,1, 3-epi-Isocucurbitacin D,2, and B,3, were isolated from the fruits ofCucurbita texana and structurally identified by UV, IR,(1)H NMR,(13)C NMR, and MS, and 2-O-Beta-glucopyranosylcucurbitacin I was identified. These compounds have not been reported previously as constituents of this species. The isolation of 3-epi-Isocucurbitacin D2 together with normal cucurbitacins suggests that isocucurbitacins occur naturally. Evidence is also discussed that isocucurbitacins are biosynthesized one step ahead of normal cucurbitacin.
[Studies on the constituents of trichosanthes root. III. Constituents of roots of Trichosanthes bracteata Voigt].[Pubmed:2760813]
Yakugaku Zasshi. 1989 Apr;109(4):265-70.
From the fresh roots of Trichosanthes bracteata Voigt., the following substances were identified: methyl palmitate, palmitic acid, suberic acid, alpha-spinasterol, stigmast-7-en-3 beta-ol, alpha-spinasterol 3-O-beta-D-glucopyranoside, stigmast-7-en-3 beta-ol 3-O-beta-D-glucopyranoside, glyceryl 1-palmitate, glyceryl 1-stearate, bryonolic acid, cucurbitacin B, isocucurbitacin B, 3-epi-isocucurbitacin B, 23,24-dihydrocucurbitacin B, 23,24-dihydroisocucurbitacin B, 23,24-dihydro-3-epi-isocucurbitacin B, cucurbitacin D, Isocucurbitacin D and D-glucose. This root contains more than 6 times cucurbitacin of the root of T. kirilowii Maxim. var. japonicum Kitam.


