MenisperineCAS# 25342-82-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25342-82-9 | SDF | Download SDF |
| PubChem ID | 161487 | Appearance | Powder |
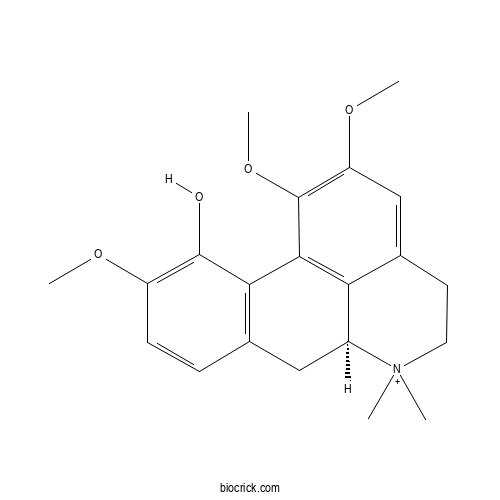
| Formula | C21H26NO4 | M.Wt | 356.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (6aS)-1,2,10-trimethoxy-6,6-dimethyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-6-ium-11-ol | ||
| SMILES | C[N+]1(CCC2=CC(=C(C3=C2C1CC4=C3C(=C(C=C4)OC)O)OC)OC)C | ||
| Standard InChIKey | XQINTCORIZHGFD-AWEZNQCLSA-O | ||
| Standard InChI | InChI=1S/C21H25NO4/c1-22(2)9-8-13-11-16(25-4)21(26-5)19-17(13)14(22)10-12-6-7-15(24-3)20(23)18(12)19/h6-7,11,14H,8-10H2,1-5H3/p+1/t14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Menisperine Dilution Calculator

Menisperine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0292 mL | 28.0584 mL | 56.1167 mL | 70.1459 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6117 mL | 7.0146 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8'-O-(3-hydroxy-3-methylglutaryl)-8'-hydroxyabscisic acid
Catalog No.:BCN0195
CAS No.:69790-31-4
- Isovitexin-2''-O-rhamnoside
Catalog No.:BCN0194
CAS No.:72036-50-1
- 9-Octadecenedioic acid
Catalog No.:BCN0193
CAS No.:4494-16-0
- 4,7-Didehydroneophysalin B
Catalog No.:BCN0192
CAS No.:134461-76-0
- Solafuranone
Catalog No.:BCN0191
CAS No.:367965-50-2
- Isomargaritene
Catalog No.:BCN0190
CAS No.:64271-11-0
- Rosmarinyl glucoside
Catalog No.:BCN0189
CAS No.:910028-78-3
- Fortunellin-6''-beta-D-glucopyranoside (Acacetin-7-O-[2''-O-rhamnosyl-6''-O-glucosyl]-glucoside)
Catalog No.:BCN0188
CAS No.:1218774-64-1
- Phloretin 3',5'-Di-C-glucoside
Catalog No.:BCN0187
CAS No.:357401-40-2
- Clemomandshuricoside B
Catalog No.:BCN0186
CAS No.:905294-48-6
- Glaucoside A
Catalog No.:BCN0185
CAS No.:81474-91-1
- 11-Deoxyisomogroside V
Catalog No.:BCN0184
CAS No.:1628293-32-2
- Batatasin V
Catalog No.:BCN0197
CAS No.:65817-45-0
- alpha-Costic acid
Catalog No.:BCN0198
CAS No.:28399-17-9
- Parishin G
Catalog No.:BCN0199
CAS No.:952283-93-1
- Arvenin III
Catalog No.:BCN0200
CAS No.:65597-45-7
- Physalin X
Catalog No.:BCN0201
CAS No.:72497-31-5
- Notoginsenoside L13
Catalog No.:BCN0202
CAS No.:2485859-56-9
- Euphorbia factor L24
Catalog No.:BCN0203
CAS No.:1613700-13-2
- Batatasin IV
Catalog No.:BCN0204
CAS No.:60347-67-3
- Cuscutamine
Catalog No.:BCN0205
CAS No.:122170-93-8
- Isocucurbitacin D
Catalog No.:BCN0206
CAS No.:68422-20-8
- 9,11-Dehydro-beta-boswellic acid
Catalog No.:BCN0207
CAS No.:471-65-8
- (3beta,22alpha)-26-(beta-glucopyranosyloxy)-22-methoxyfurost-5-en-3-yl 2-O-(6-deoxy-alpha-mannopyranosyl)-beta-glucopyranosiduronic acid
Catalog No.:BCN0208
CAS No.:107783-53-9
Chemical profile and potential mechanisms of Huo-Tan-Chu-Shi decoction in the treatment of coronary heart disease by UHPLC-Q/TOF-MS in combination with network pharmacology analysis and experimental verification.[Pubmed:33992976]
J Chromatogr B Analyt Technol Biomed Life Sci. 2021 Jun 15;1175:122729.
Huo-Tan-Chu-Shi Decoction (HTCSD), a traditional Chinese medicine (TCM) prescription within Guangdong Provincial TCM Hospital (the largest TCM hospital in China), is used for effective clinical treatment of coronary heart disease (CHD) caused by phlegm-dampness syndrome with high incidence in the hot and humid climate of Lingnan region. However, its chemical components responsible for the therapeutic effects remain unclear, which restricts its application and further development. Hence, a detailed workflow, combing with UHPLC-Q/TOF-MS, network pharmacology analysis and experimental verification, was proposed and applied to characterize the chemical profile and potential mechanism of HTCSD against CHD. As a result, a total of 130 components from all six composed herbal medicines were characterized in a rapid and sensitive manner through UHPLC-Q/TOF-MS, of which 33 compounds were unambiguously confirmed with reference standards. Consequently, based on the integrated pharmacology network of "herbs-chemicals-targets-pathways-therapeutic effects", four chemicals (magnoflorine, Menisperine, 13-hydroxyberberine, luteolin) with four CHD related targets (SRC, MAPK1, EGFR and AKT1) were considered as the key components and targets of HTCSD in the treatment of CHD. Furthermore, the effect of HTCSD was confirmed in animal experiments by enhancing the phosphorylation of MAPK, and the published literature and molecular binding results suggested that magnoflorine and luteolin tended to be the critical compounds involved in the process. Taken together, the characterization of chemical profile combined with network pharmacology analysis and experimental verification not only provided an efficient insight into the overall chemical profile of HTCSD but also revealed the potential pharmacological components and mechanisms of HTCSD against CHD, which laid a necessary chemical and biological basis for the discovery of in vivo bioactive components and the further revelation of functionary mechanism.
Rapid profiling of alkaloid analogues in Sinomenii Caulis by an integrated characterization strategy and quantitative analysis.[Pubmed:31202880]
J Pharm Biomed Anal. 2019 Sep 10;174:376-385.
Alkaloids, the principal constituents in the caulis of Sinomenium acutum, have gained an increasing interest over the past decades since they are widely employed as a clinical treatment for rheumatoid arthritis. In the present study, an integrated characterization strategy by combining mass defect filtering-based structure classification (MDFSC) and diagnostic fragment-ion-based extension (DFIBE) was firstly proposed for rapid profiling of alkaloids in Sinomenii Caulis (SC) via ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS). The rectangular MDFSC window could more accurately screen the target alkaloids of different types, and the DFIBE could facilitate the acquisition of characteristic fragment ions for structural elucidation of alkaloids. High-performance liquid chromatography (HPLC) fingerprints with principal component analysis (PCA) and hierarchical clustering analysis (HCA) was established for identifying the chemical markers and simultaneous determination of sinomenine, magnoflorine, Menisperine, stepharanine and ehydrodiscretine. A total of 91 alkaloids, including 82 targeted ones (26 morphinans, 22 aporphines, 20 protoberberines and 14 benzylisoquinolines) were unambiguously identified or tentatively characterized in SC, and 14 of them were reported for the first time. Sinomenine and magnoflorine could be selected as chemical markers to evaluate the quality of SC from different localities. In conclusion, the proposed method provided a potential approach for chemical profiling and holistic quality control of herbal medicines.
Identification of anti-inflammatory components in Sinomenii Caulis based on spectrum-effect relationship and chemometric methods.[Pubmed:30738242]
J Pharm Biomed Anal. 2019 Apr 15;167:38-48.
The present study aimed to identify the anti-inflammatory components in Sinomenii Caulis (SC) based on spectrum-effect relationship and chemometric methods. A phytochemical investigation of SC extract was performed firstly and afforded eleven potential bioactive compounds. The HPLC fingerprints of 19 batches of SC samples were evaluated by the chemometric methods such as similarity analysis (SA) and hierarchical clustering analysis (HCA). The anti-inflammatory effects of these samples were determined by inhibition of Nitric Oxide (NO) production. Partial least squares regression (PLSR) and artificial neural network (ANN) were used to explore the spectrum-effect relationship of SC. The results indicated that there was a close correlation between chemical fingerprint and anti-inflammatory activity of SC, and peaks 8, 9, 12, 13, 14, 16, 19 and 22 might be potential anti-inflammatory compounds in SC. The verification experiments by testing individual compounds and a combination of them indicated that sinomenine (P8), magnoflorine (P13), Menisperine (P16) and stepharanine (P19) were the major anti-inflammatory compounds in SC. Collectively, the present study established the spectrum-effect relationship mode of SC and discovered the anti-inflammatory compounds in SC, which could be used for exploration of bioactive components and quality control of herbal medicines.
Capillary-HPLC with tandem mass spectrometry in analysis of alkaloid dyestuffs - a new approach.[Pubmed:29124775]
Electrophoresis. 2018 May;39(9-10):1276-1283.
Development of the identification method of alkaloid compounds in Amur cork tree as well as not examined so far Oregon grape and European Barberry shrubs are presented. The novel approach to separation of alkaloids was applied and the capillary-high-performance liquid chromatography (capillary-HPLC) system was used, which has never previously been reported for alkaloid-based dyestuffs analysis. Its optimization was conducted with three different stationary phases (unmodified octadecylsilane-bonded silica, octadecylsilane modified with polar groups and silica-bonded pentaflourophenyls) as well as with different solvent buffers. Detection of the isolated compounds was carried out using diode-array detector (DAD) and tandem mass spectrometer with electrospray ionization (ESI MS/MS). The working parameters of ESI were optimized, whereas the multiple reactions monitoring (MRM) parameters of MS/MS detection were chosen based on the product ion spectra of the quasi-molecular ions. Calibration curve of berberine has been estimated (y = 1712091x + 4785.03 with the correlation coefficient 0.9999). Limit of detection and limit of quantification were calculated to be 3.2 and 9.7 ng/mL, respectively. Numerous alkaloids (i.e., berberine, jatrorrhizine and magnoflorine, as well as phellodendrine, Menisperine and berbamine) were identified in the extracts from alkaloid plants and silk and wool fibers dyed with these dyestuffs, among them their markers.
Analysis of six active components in Radix tinosporae by nonaqueous capillary electrophoresis with mass spectrometry.[Pubmed:28975733]
J Sep Sci. 2017 Dec;40(23):4628-4635.
Nonaqueous capillary electrophoresis with mass spectrometry has advantages for the analysis of active components in herbs. Here, a rapid nonaqueous capillary electrophoresis with mass spectrometry method was developed to separate, identify, and quantify palmatin, columbin, cepharanthine, Menisperine, magnoflorine, and 20-hydroxyecdysone in Radix tinosporae. Electrospray ionization MS(1-3) spectra of the six components were collected and possible cleavage pathways of main fragment ions were elucidated. The conditions that could affect separation, such as the composition of running buffer and applied voltage, were studied, and the conditions that could affect the mass spectrometry detection, such as the composition and flow rate of sheath liquid, the pressure of nitrogen gas, and the temperature and flow rate of the dry gas, were also optimized. Under the optimized conditions, the correlation coefficient was >0.99. The relative standard deviations of migration time and peak areas were <10%. The recoveries were calculated to be 99.31-107.80% in real samples. It has been demonstrated that the proposed method has good potential to be applied to determine the six bioactive components in Radix tinosporae.
Qualitative and quantitative analysis of chemical constituents of Ptychopetalum olacoides Benth.[Pubmed:28750557]
Nat Prod Res. 2018 Feb;32(3):354-357.
Ptychopetalum olacoides is a folk medicinal plant for health care in market, especially in Brazil. Fourteen known compounds were isolated from P. olacoides and their chemical structures were elucidated by extensive spectroscopic data, including 1D NMR, 2D NMR, UV, IR and HR-ESI-MS. The 14 known compounds were identified as N-trans-feruloyl-3,5-dihydroxyindolin-2-one (1), magnoflorine (2), Menisperine (3), 4-coumaroylserotonin (4), moschamine (5), luteolin (6), 4'-methoxyluteolin (7), 3-methoxyluteolin (8), 3, 7-dimethoxyluteolin (9), caffeic acid (10), ferulic acid (11), vanillic acid (12), syringic acid (13) and ginsenoside Re (14). To our knowledge, compounds (1-6, 13-14) were isolated from the plant for the first time. Additionally, quantitative analysis results indicated that calibration equations of compounds (1-3, 6, 9, 11-13) exhibited good linear regressions within the test ranges (R(2) >/= 0.9990) and magnoflorine and Menisperine were the major constituents in the barks of P. olacoides. The contents of magnoflorine and Menisperine accounted for 75.96% of all analytes. However, the content of phenolic components was smaller and the highest content was no more than 1.04 mg/g. Collectively, these results suggested that alkaloids are the dominant substances in P. olacoides, which can make a difference for the quality control and further use of P. olacoides.
Screening and identification of Caulis Sinomenii bioactive ingredients with dual-target NF-kappaB inhibition and beta2- AR agonizing activities.[Pubmed:27187693]
Biomed Chromatogr. 2016 Nov;30(11):1843-1853.
Caulis Sinomenii (CS) is a valuable traditional medicine in China. Its extract can act as an anti-inflammatory agent and a vascular smooth muscle relaxant. However, the underlying mechanisms remain unknown. In this study, we developed a simple dual-target method based on ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry combined with a dual-target bioactive screening assay for anti-inflammatory and antispasmodic activities to characterize the chemical structure of various bioactive compounds of CS rapidly. Seven potential NF-kappaB inhibitors were identified, including laudanosoline-1-O-xylopyranose, 6-O-methyl-laudanosoline-1-O-glucopyranoside, Menisperine, sinomenine, laurifoline, magnoflorine and norsinoacutin. Furthermore, IL-6 and IL-8 assays confirmed the anti-inflammatory effects of these potential NF-kappaB inhibitors, in which laudanosoline-1-O-d-xylopyranose and Menisperine were revealed as novel NF-kappaB inhibitors. Among the seven identified alkaloids, three potential beta2 -adrenergic receptor agonists, including sinomenine, magnoflorine and laurifoline, were characterized using a luciferase reporter system to measure for the activity of beta2 -adrenergic receptor agonists. Finally, sinomenine, magnoflorine and laurifoline were identified not only as potential NF-kappaB inhibitors but also as potential beta2 -adrenegic receptor agonists, which is the first time this has been reported. Molecular dynamic simulation and docking results suggest that the three dual-bioactive constituents could not only inhibit Pseudomonas aeruginosa PAK strain-induced inflammatory responses via a negative regulation of the Braf protein that participates in MAPK signaling pathway but also activate the beta2 -adrenegic receptor. These results suggest that CS extract has dual signaling activities with potential clinical application as a novel drug for asthma.
Simultaneous detection of eight active components in Radix Tinosporae by ultra high performance liquid chromatography coupled with electrospray tandem mass spectrometry.[Pubmed:27059766]
J Sep Sci. 2016 Jun;39(11):2036-42.
A rapid and sensitive ultra high performance liquid chromatography with electrospray ionization tandem mass spectrometry method was developed and validated for the simultaneous determination of eight major active components (magnoflorine, Menisperine, 20-hydroxyecdysone, cepharanthine, columbamine, jatrorrhizine, columbin, and palmatine) in Radix Tinosporae. The separation was performed on an InterSustainSwift C18 column (1.9 mum, 2.1 id x 100 mm) at 40 degrees C with a gradient elution. A mixture of acetonitrile and methanol (v/v = 1:1) and ammonium acetate buffer (25 mmol/L ammonium acetate with 0.2% formic acid) were used as mobile phases, and the flow rate was set at 0.4 mL/min. The recovery was tested in real samples and calculated to be 86.97-111.28%, and all the compounds showed good linearity (r > 0.998) in relatively wide concentration ranges. The developed method was applied to the determination of eight active compounds in real herb samples, which were collected from four different places. It has been demonstrated that the proposed method has great potential for the quality control of the traditional Chinese medicine Radix Tinosporae.
Tissue-specific metabolite profiling of alkaloids in Sinomenii Caulis using laser microdissection and liquid chromatography-quadrupole/time of flight-mass spectrometry.[Pubmed:22721764]
J Chromatogr A. 2012 Jul 27;1248:93-103.
Secondary metabolites accumulated in different tissues and cells of herbs are usually bioactive components of herbal medicines. Thus, tissue- and cell-specific phytochemical profiling should be useful for indicating relationship between herbal tissues and chemicals, and evaluating the quality of a medicinal herb. Here, a method that combining laser microdissection and ultra-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry (LMD with UPLC-Q/TOF-MS) was established to achieve simultaneous localization and determination of bioactive components in herbal medicines. Sinomenii Caulis, sourced from the stems of Sinomenium acutum (Thunb.) Rehd. et Wils., was set as an illustrative case, and its phytochemicals were profiled by the present method through analyses of different microdissected tissues and cells, involving epidermis, cortex, stone cells, pericycle, vascular bundles and pith. Results revealed that different tissues and cells contained varied alkaloids, among which six alkaloids, i.e. 6-Me-ether-12-O-beta-D-glucopyranoside-laudanosoline (peak 4), sinomenine (peak 6), N-norsinoacutine (peak 7), magnoflorine (peak 11), laurifoline (peak 16) and Menisperine (peak 17) were detected in all microdissected parts, and sinomenine and magnoflorine were the two most abundant components. By further quantitative determination, alkaloids were generally demonstrated to distribute in the outer part of the cortex, phloem and xylem. According to the relationship between alkaloids and tissues revealed in our study, Sinomenii Caulis of larger diameter has proportionately more bioactive components, and is therefore of higher quality for medicinal use. The method of LMD with UPLC-Q/TOF-MS developed in this study was initially applied to the research of medicinal herbs, and proved to be high sensitive, low cost, convenient and practical.
Chemical fingerprint analysis of Phellodendri Amurensis Cortex by ultra performance LC/Q-TOF-MS methods combined with chemometrics.[Pubmed:21049522]
J Sep Sci. 2010 Nov;33(21):3347-53.
Ultra performance LC with quadrupole TOF MS (UPLC/Q-TOF-MS) fingerprinting is first developed for the identification of the major components of Phellodendri Amurensis Cortex (PAC). The PAC samples are separated using a Waters ACQUITY UPLC BEH C18 (2.1x50 mm, 1.7 mum) by linear gradient elution using water (containing 0.2% formic acid) and acetonitrile (containing 0.2% formic acid) as the mobile phase. Ten batches of PAC are selected to construct the UPLC/Q-TOF-MS fingerprint. Sixteen common peaks in the fingerprint are obtained, ten of which are tentatively identified, with reference to the literature data, as phellodendrine, magnoflorine, tetrahydropjatrorrhizine, Menisperine, tetrahydropalmatine, jatrorrhizine, palmatine, berberine, obacunone, and limonin. Chemometric methods are also employed to evaluate the variation of herbal drugs and other closely related herbs based on the characteristics of peaks in the UPLC/Q-TOF-MS profiles. The developed fingerprint assay is a powerful method that may be used to conduct quality control of PAC.
Genetic and chemical comparison of Boi (Sinomeni Caulis et Rhizoma) and Seifuto (Caulis Sinomenii).[Pubmed:20217263]
J Nat Med. 2010 Jul;64(3):257-65.
Boi and its original plant Sinomenium acutum from Japan were compared with Seifuto and its botanical origins from China in terms of their internal transcribed spacer (ITS) sequences and major chemical components. Boi, Seifuto, and their botanical origins overall showed seven variable sites in the ITS sequence and six genotypes. Japanese S. acutum and Boi had one nucleotide variation at position 593 to show two genotypes (J1 and J2) and their heterozygote (J3). Seifuto samples and their botanical origins, S. acutum and S. acutum var. cinereum from China, showed three genotypes (C1, C2, and C3), which did not agree with the botanical classification, indicating that they cannot be distinguished according to their ITS sequences. All Seifuto samples from Henan market showed the same ITS genotype (C1). The Japanese and Chinese genotypes differed in the nucleotide position 424, which can be used to distinguish the country of origin of these materials. In the HPLC analysis of six major components, sinomenine (1), magnoflorine (2), Menisperine (3), 6-O-methyllaudanosoline glucoside (4), liriodendrin (5), and menisdaurin (6), all were detected in Boi, whereas five (all except for menisdaurin) were detected in Seifuto. The main component in the rhizome of Seifuto was sinomenine, whereas magnoflorine was the main component in the rhizome and the climbing stem of Boi. The content of sinomenine in Seifuto was almost twice that in Boi. Although the individual content of alkaloids 1-4 differed between Boi and Seifuto, the total contents of these alkaloids were comparable between them both in the climbing stem and rhizome.
Identification of major alkaloids and steroidal saponins in rat serum by HPLC-diode array detection-MS/MS following oral administration of Huangbai-Zhimu herb-pair Extract.[Pubmed:18318017]
Biomed Chromatogr. 2008 Aug;22(8):835-50.
Huangbai-Zhimu herb-pair (HBZMHP) is a widely used Chinese traditional medicine formula in treating various diseases; however, its active components have remained unknown. In this paper, serum chemistry and combined high-performance liquid chromatography (HPLC), diode-array detection and mass-spectrometry (MS) techniques were used to study the constituents of HBZMHP extract absorbed into rat serum after oral administration. A total of nine characteristic HPLC peaks in the TIC chromatograms were identified as magnoflorine (1), Menisperine (2), palmatine (3), berberine (4), timosaponin N or timosaponin E1 (5), timosaponin D (6), timosaponin BIII, anemarsaponin C or xilingsaponin B (7) timosaponin BII (8) and timosaponin AIII (9). All of the identified peaks were constituents of HBZMHP extract. The results narrow the range of active compounds to be found in HBZMHP extract, and pave the way for the follow-up action mechanism research.
In vivo analysis and spatial profiling of phytochemicals in herbal tissue by matrix-assisted laser desorption/ionization mass spectrometry.[Pubmed:17313187]
Anal Chem. 2007 Apr 1;79(7):2745-55.
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) was developed for spatial profiling of phytochemicals and secondary metabolites in integrated herbal tissue without solvent extraction. Abundant alkaloid ions, including (+)-Menisperine (m/z 356), magnoflorine (m/z 342), stepharanine (m/z 324), protonated sinomenine (m/z 330), protonated sinomendine (m/z 338), and a metabolite at m/z 314, could be directly desorbed from alpha-cyano-4-hydroxycinnamic acid- (CHCA-) coated stem tissue of Sinomenium acutum upon N2 laser (337 nm) ablation, while the ion signals desorbed from sinapinic acid- (SA-) coated and 2,5-dihydroxybenzoic acid- (DHB-) coated stem tissue were at least 10 times weaker. Solvent composition in the matrix solution could have significant effects on the ion intensity of the metabolites. Under optimized conditions that maximize the ion intensity and form homogeneous matrix crystals on the tissue surface, spatial distributions of the metabolites localized in different tissue regions, including cortex, phloem, xylem, rim, and pith, and their relative abundances could be semiquantitatively determined. The three metabolites detected at m/z 356, 342, and 314 showed specific distributions in the herbal samples collected from different growing areas, while others were not. By applying principal component analysis (PCA), the characteristic metabolites in specific tissue regions could be easily determined, allowing unambiguous differentiation of the herbal samples from different geographic locations.
Simultaneous determination of eight components in Radix Tinosporae by high-performance liquid chromatography coupled with diode array detector and electrospray tandem mass spectrometry.[Pubmed:17084577]
J Pharm Biomed Anal. 2007 Feb 19;43(3):994-9.
High-performance liquid chromatography (HPLC) coupled with electrospray tandem mass spectrometry (ESI-MS-MS) and diode array detection (DAD) was used to identify and simultaneously determine eight major ingredients in Radix Tinosporae. The assay was performed on a Diamonsil C(18) analytical column with a gradient solvent system of A (water containing 0.2% formic acid, 20mM ammonium acetate) and B (methanol/acetonitrile=1/1, v/v). The 217, 248, 270 and 347 nm, respectively, were chosen as the monitoring wavelengths to determine four structural types of components, say columbin, phytoecdysteroids (including 20-hydroxyecdysone, 2-deoxy-20-hydroxyecdysone 3-O-beta-d-glucopyranoside and 2-deoxy-20-hydroxyecdysone), Menisperine and protoberberine alkaloids (including columbamine, jatrorrhizine and palmatine). This method was validated in respect to precision, repeatability and accuracy, and was successfully applied to quantify the eight components in 39 batches of R. Tinosporae for quality control purpose. The results indicated that the proposed method could be readily utilized as a quality control method for traditional Chinese medicine (TCM).
Quaternary isoquinoline alkaloids from Xylopia parviflora.[Pubmed:15081299]
Phytochemistry. 2004 Apr;65(7):939-44.
From the quaternary alkaloidal fraction of the bark and the root of Xylopia parviflora (Annonaceae), four isoquinoline alkaloids, xylopinidine, dehydrocoreximine, N, N-dimethylanomurine and N-methylphoebine were isolated along with the known compounds, pycnarrhine, lotusine, 6,7-dimethoxy-2-methyl-isoquinolinium salt, 1,2-dehydroreticuline, (-)-phellodendrine, (+)-tembetarine, (-)-litcubine, (+)-magnoflorine, tetradehydroreticuline, (-)-oblongine, (+)-Menisperine, (+)-N-methylcorydine, stepharanine, (+)-xanthoplanine, dehydrodiscretine, jatrorrhizine and palmatine. 3,4-Dihydro-6,7-dimethoxy-2-methyl-isoquinolinium and N-methylpurpuerine were isolated as natural products for the first time. Their structures were determined on the basis of spectroscopic evidence.


