RicinineCAS# 524-40-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 524-40-3 | SDF | Download SDF |
| PubChem ID | 10666 | Appearance | Powder |
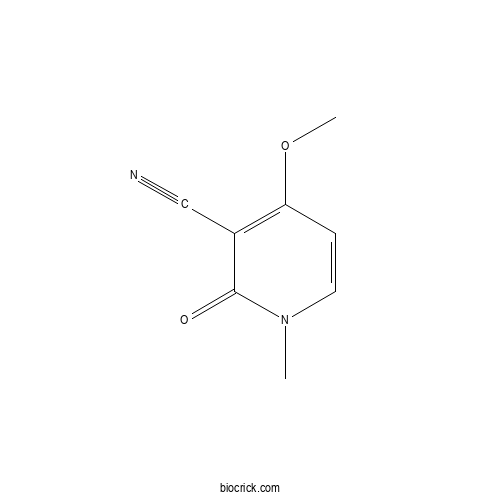
| Formula | C8H8N2O2 | M.Wt | 164.16 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-methoxy-1-methyl-2-oxopyridine-3-carbonitrile | ||
| SMILES | CN1C=CC(=C(C1=O)C#N)OC | ||
| Standard InChIKey | PETSAYFQSGAEQY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8N2O2/c1-10-4-3-7(12-2)6(5-9)8(10)11/h3-4H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ricinine Dilution Calculator

Ricinine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0916 mL | 30.4581 mL | 60.9162 mL | 121.8324 mL | 152.2904 mL |
| 5 mM | 1.2183 mL | 6.0916 mL | 12.1832 mL | 24.3665 mL | 30.4581 mL |
| 10 mM | 0.6092 mL | 3.0458 mL | 6.0916 mL | 12.1832 mL | 15.229 mL |
| 50 mM | 0.1218 mL | 0.6092 mL | 1.2183 mL | 2.4366 mL | 3.0458 mL |
| 100 mM | 0.0609 mL | 0.3046 mL | 0.6092 mL | 1.2183 mL | 1.5229 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-O-Methylcatalpol
Catalog No.:BCN0223
CAS No.:1617-84-1
- Linaroside
Catalog No.:BCN0222
CAS No.:53452-12-3
- Tubuloside B
Catalog No.:BCN0221
CAS No.:112516-04-8
- Corysamine chloride
Catalog No.:BCN0220
CAS No.:11028-77-6
- Coryneine
Catalog No.:BCN0219
CAS No.:7224-66-0
- 3',5-Di-O-methyl quercetin
Catalog No.:BCN0218
CAS No.:40554-94-7
- 3,5,6,7,8,4'-hexamethoxyflavone
Catalog No.:BCN0217
CAS No.:34170-18-8
- Rutarensin
Catalog No.:BCN0216
CAS No.:119179-04-3
- 4-Methyl-6-phenyl-2H-pyranone
Catalog No.:BCN0215
CAS No.:4467-30-5
- Physalin F
Catalog No.:BCN0214
CAS No.:57423-71-9
- 3-Oxo-olean-12-en-28-oic acid methyl ester
Catalog No.:BCN0213
CAS No.:1721-58-0
- 7-Ketocholesterol
Catalog No.:BCN0212
CAS No.:566-28-9
- Quercetin 3-O-malonylglucoside
Catalog No.:BCN0225
CAS No.:96862-01-0
- Luteolin-7-O-alpha-L-arabinopyranosyl (1->6)-beta-D-glucopyranoside
Catalog No.:BCN0226
CAS No.:52714-82-6
- Emodin 6-O-beta-D-glucoside
Catalog No.:BCN0227
CAS No.:34298-85-6
- 24-Methylene cycloartanyl ferulate
Catalog No.:BCN0228
CAS No.:469-36-3
- Valtrate Hydrine B4
Catalog No.:BCN0229
CAS No.:18296-48-5
- Polygalasaponin II
Catalog No.:BCN0230
CAS No.:162857-62-7
- L-Gamma-Glutamyl-S-[(4-hydroxyphenyl)methyl]-L-cysteinylglycine
Catalog No.:BCN0231
CAS No.:129636-38-0
- 2-Naphthalenecarboxylic acid, 4-(D-glucopyranosyloxy)-1-hydroxy-3-(3-hydroxy-3-methylbutyl)-, methyl ester
Catalog No.:BCN0232
CAS No.:125906-48-1
- 1-Methylhydantoin
Catalog No.:BCN0233
CAS No.:616-04-6
- Echinocystic acid 28-O-beta-D-glucoside
Catalog No.:BCN0234
CAS No.:99633-30-4
- Fissistigine A
Catalog No.:BCN0235
CAS No.:70420-58-5
- 16,25-Diacetate cyclosiversioside F
Catalog No.:BCN0236
CAS No.:452919-90-3
Further development of a liquid chromatography-high-resolution mass spectrometry/mass spectrometry-based strategy for analyzing eight biomarkers in human urine indicating toxic mushroom or Ricinus communis ingestions.[Pubmed:34080326]
Drug Test Anal. 2021 Jun 3.
Recently, we presented a strategy for analysis of eight biomarkers in human urine to verify toxic mushroom or Ricinus communis ingestions. However, screening for the full panel is not always necessary. Thus, we aimed to develop a strategy to reduce analysis time and by focusing on two sets of analytes. One set (A) for biomarkers of late-onset syndromes, such as phalloides syndrome or the syndrome after castor bean intake. Another set (B) for biomarkers of early-onset syndromes, such as pantherine-muscaria syndrome and muscarine syndrome. Both analyses should be based on hydrophilic-interaction liquid chromatography coupled with high-resolution mass spectrometry (MS)/MS (HILIC-HRMS/MS). For A, urine samples were prepared by liquid-liquid extraction using dichloromethane and subsequent solid-phase extraction of the aqueous supernatant. For B urine was precipitated using acetonitrile. Method A was validated for Ricinine and alpha- and beta-amanitin and method B for muscarine, muscimol, and ibotenic acid according to the specifications for qualitative analytical methods. In addition, robustness of recovery and normalized matrix factors to matrix variability measured by urinary creatinine was tested. Moreover, applicability was tested using 10 urine samples from patients after suspected mushroom intoxication. The analytes alpha- and beta-amanitin, muscarine, muscimol, and ibotenic acid could be successfully identified. Finally, psilocin-O-glucuronide could be identified in two samples and unambiguously distinguished from bufotenine-O-glucuronide via their MS(2) patterns. In summary, the current workflow offers several advantages towards the previous method, particularly being more labor-, time-, and cost-efficient, more robust, and more sensitive.
Ricicomin A, a new alkaloid from the leaves of Ricinus communis Linn.[Pubmed:33096957]
Nat Prod Res. 2020 Oct 23:1-7.
From the leaves of Ricinus communis Linn., one new alkaloid, named ricicomin A (1) together with three known ones, Ricinine (2), N-demethylRicinine (3) and 4-[2-formyl-5-(methoxymethyl)-1H-pyrrol-1-yl]butanoic acid (4) were justified by repeated chromatographic methods. Their structures were determined by comprehensive IR, HR-ESI-MS and NMR analyses. Compound 4 was identified for the first time from the genus Ricinus. DFT-NMR chemical shift calculations and subsequent DP4+ probability methods were applied to confirm the chemical structure of 1. Compounds 1-3 did not display cytotoxic effect against three human cancer cell lines (MCF-7, HepG2 and HeLa) using SRB assay.
Non-Lethal Intoxication by Ingestion of 50 Castor Beans: Serial Measurement of Ricinine in Blood, Plasma and Urine.[Pubmed:32991682]
J Anal Toxicol. 2021 May 14;45(5):e8-e12.
A 30-year-old woman presented to the emergency department 2 days after ingestion of 50 castor beans. Her symptoms on admission were vomiting, diarrhea, abdominal cramps, agitation and anxiety. Initial laboratory tests showed a slightly elevated C-reactive protein and mild liver and kidney dysfunction. The patient was transferred to the medium care unit of our hospital where she was observed for possible organ failure. During the next days, the kidney function improved and liver function started to recover. Four days after admission, the patient was transferred to the psychiatric ward. Urine, serum, plasma and whole-blood samples were analyzed for Ricinine using a quantitative LC-MS-MS method. Initial values on admission (serum and urine) were very high in comparison with previously reported cases. Based on these values, the patient was monitored closely in the following days. The patient made a full recovery, and during the course of hospitalization, concentrations of Ricinine in plasma/serum, blood and urine gradually declined. The presence of Ricinine in a patient's blood or plasma is a proof of castor bean and, hence, ricin exposure. However, based on this case and previously reported cases in literature, we can conclude that no clear correlation can be established between Ricinine blood, plasma or urine levels and the severity of the intoxication. Clinicians should be aware of the potential danger of a ricin intoxication, and patients should be monitored closely for several days due to the unpredictable outcome of the intoxication.
Fatal intoxication by intravenous injection of castor bean (Ricinus communis L.) extract-a case study.[Pubmed:32548760]
Int J Legal Med. 2020 Nov;134(6):2133-2141.
A case report of a 25-year-old man who committed suicide by intravenous injection himself of an aqueous home-made castor bean extract is presented. The patient was hospitalized and treated symptomatically and was released at its own request fourth day after intoxication. The next day, the patient's condition deteriorated, and he died 6 days after intoxication even though he was given medical care. Case history, autopsy, and toxicological investigation of ante- and post-mortem collected materials are described. Blood and urine collected from the patient ante-mortem and other several biological materials (namely blood from the upper and lower limb, blood from the right and left ventricle, pericardial fluid, vitreous humour, liver, kidney, and spleen) were collected post-mortem during autopsy. Liquid-liquid extraction procedure followed by high-performance liquid chromatography tandem mass spectrometry analysis for identification and determination of Ricinine as a biomarker of ricin/castor seed intoxication was developed and validated. The method was applied on analysis of collected ante- and post-mortem biological materials. The post-mortem contents of Ricinine in organs (namely the liver, kidney, and spleen) are firstly reported. The obtained results indicated approximately uniform distribution of Ricinine (concentration level about 1 ng mL(-1)) in the body after death. In addition, the GC-MS method was also applied for the analysis of extract of castor seed and the patient's urine, to demonstrate alternative possibility for identification of Ricinine for clinical and forensic purposes.
Isolation, characterization, and hepatoprotective properties of betulinic acid and ricinine from Tetracarpidium conophorum seeds (Euphorbiaceae).[Pubmed:32529649]
J Food Biochem. 2021 Mar;45(3):e13288.
The present study is to isolate and characterize betulinic acid and Ricinine from T. conophorum seeds. Phytochemical investigation on hexane fraction of T. conophorum seeds led to the isolation of two compounds, Betulinic acid (1), and Ricinine (2). Betulinic acid and Ricinine were screened against HepG2 cells and tested in vivo in CCl4 -induced experimental rats model. Results from this study showed that the compounds had hepatoprotective and cytotoxic activities. It was observed that betulinic acid inhibited HepG2 cell with percentage inhibition of 54% compared with standard doxorubicin (64%), while Ricinine was inactive against HepG2 cell lines. Furthermore, molecular docking was carried out on betulinic acids and Ricinine, with binding energies of -11.2 kcal/mol and -5.4 kcal/mol, respectively, indicating strong binding sites and interactions with Hepatitis B Virus DNA polymerase. Therefore, findings from this study suggest that betulinic acid possess cytotoxic and hepatoprotective properties, while Ricinine exhibited hepatoprotection in CCl4 -induced liver damage. PRACTICAL APPLICATIONS: Medicinal plants contain unrestricted ability to make compounds that intrigue researchers in the quest for novel phyto-therapeutic drugs. The continuous exploration of new compounds in the medicinal plant is an auspicious strategy for the prevention of diseases. Therefore, the purpose of this research is to evaluate the cytotoxic and hepatoprotective compounds (betulinic acid and Ricinine) isolated from T. conophorum seeds.
Near-fatal poisoning after ricin injection.[Pubmed:32475181]
Clin Toxicol (Phila). 2021 Feb;59(2):158-168.
OBJECTIVE: To report a near-fatal poisoning after intentional injection of ricin from a castor bean (Ricinus communis) extract. CASE REPORT: A 21 year-old man self-injected approximately 3 mL of a castor bean extract intramuscularly and subcutaneously in the left antecubital fossa. Upon admission to our ED (1 h post-exposure; day 1, D1) he was awake and alert, but complained of mild local pain and showed slight local edema and erythema. He evolved to refractory shock ( approximately 24 h post-exposure) that required the administration of a large volume of fluids and high doses of norepinephrine and vasopressin, mainly from D2 to D4. During this period, he developed clinical and laboratory features compatible with systemic inflammatory response syndrome, multiple organ dysfunction, capillary leak syndrome, rhabdomyolysis, necrotizing fasciitis and possible compartment syndrome. The patient underwent forearm fasciotomy on D4 and there was progressive improvement of the hemodynamic status from D7 onwards. Wound management involved several debridements, broad-spectrum antibiotics and two skin grafts. Major laboratory findings within 12 days post-exposure revealed hypoalbuminemia, proteinuria, thrombocytopenia, leukocytosis and increases in cytokines (IL-6, IL-10 and TNF-alpha), troponin and creatine kinase. Ricin A-chain (ELISA) was detected in serum up to D3 (peak at 24 h post-exposure), with approximately 79% being excreted in the urine within 64 h post-exposure. Ricinine was detected in serum and urine by LC-MS up to D5. A ricin A-chain concentration of 246 microg/mL was found in the seed extract, corresponding to the injection of approximately 738 microg of ricin A-chain ( approximately 10.5 microg/kg). The patient was discharged on D71, with limited range of motion and function of the left forearm and hand. CONCLUSION: Ricin injection resulted in a near-fatal poisoning that evolved with septic shock-like syndrome, multiple organ dysfunction and necrotizing fasciitis, all of which were successfully treated with supportive care.
The Effects of Gamma Irradiation on Chemical Biomarker Recovery from Mixed Chemical/Biological Threat Exposure Specimens.[Pubmed:32445395]
J Appl Lab Med. 2020 Mar 1;5(2):273-280.
BACKGROUND: Irradiative sterilization of clinical specimens prior to chemical laboratory testing provides a way to not only sterilize pathogens and ensure laboratorian safety but also preserve sample volume and maintain compatibility with quantitative chemical diagnostic protocols. Since the compatibility of clinical biomarkers with gamma irradiation is not well characterized, a subset of diagnostic biomarkers ranging in molecular size, concentration, and clinical matrix was analyzed to determine recovery following gamma irradiation. METHODS: Sample irradiation of previously characterized quality control materials (QCs) at 5 Mrad was carried out at the Gamma Cell Irradiation Facility at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. Following irradiation, the QCs were analyzed alongside non-irradiated QCs to determine analyte recovery between dosed and control samples. RESULTS: Biomarkers for exposure to abrin, ricin, and organophosphorus nerve agents (OPNAs) were analyzed for their stability following gamma irradiation. The diagnostic biomarkers included adducts to butyrylcholinesterase, abrine, and Ricinine, respectively, and were recovered at over 90% of their initial concentration. CONCLUSIONS: The results from this pilot study support the implementation of an irradiative sterilization protocol for possible mixed-exposure samples containing both chemical and biological threat agents (mixed CBTs). Furthermore, irradiative sterilization significantly reduces a laboratorian's risk of infection from exposure to an infectious agent without compromising chemical diagnostic testing integrity, particularly for diagnostic assays in which the chemical analyte has been shown to be fully conserved following a 5 Mrad irradiative dose.
Development and application of a strategy for analyzing eight biomarkers in human urine to verify toxic mushroom or ricinus communis ingestions by means of hydrophilic interaction LC coupled to HRMS/MS.[Pubmed:32200933]
Talanta. 2020 Jun 1;213:120847.
The analytical proof of a toxic mushroom and/or plant ingestion at an early stage of a suspected intoxication can be crucial for fast therapeutic decision making. Therefore, comprehensive analytical procedures need to be available. This study aimed to develop a strategy for the qualitative analysis of alpha- and beta-amanitin, psilocin, bufotenine, muscarine, muscimol, ibotenic acid, and Ricinine in human urine by means of hydrophilic interaction liquid chromatography-high resolution MS/MS (HILIC-HRMS/MS). Urine samples were prepared by hydrophilic-phase liquid-liquid extraction using dichloromethane and subsequent solid-phase extraction and precipitation, performed in parallel. Separation and identification of the biomarkers were achieved by HILIC using acetonitrile and methanol as main eluents and Orbitrap-based mass spectrometry, respectively. The method was validated as recommended for qualitative procedures and tests for selectivity, carryover, and extraction recoveries were included to also estimate the robustness and reproducibility of the sample preparation. Limits of identification were 1 ng/mL for alpha- and beta-amanitin, 5 ng/mL for psilocin, bufotenine, muscarine, and Ricinine, and 1500 ng/mL and 2000 ng/mL for ibotenic acid and muscimol, respectively. Using gamma-amanitin, l-tryptophan-d5, and psilocin-d10 as internal standards, compensation for variations of matrix effects was shown to be acceptable for most of the toxins. In eight urine samples obtained from intoxicated individuals, alpha- and beta-amanitin, psilocin, psilocin-O-glucuronide, muscimol, ibotenic acid, and muscarine could be identified. Moreover, psilocin-O-glucuronide and bufotenine-O-glucuronide were found to be suitable additional targets. The analytical strategy developed was thus well suited for analyzing several biomarkers of toxic mushrooms and plants in human urine to support therapeutic decision making in a clinical toxicology setting. To our knowledge, the presented method is by far the most comprehensive approach for identification of the included biomarkers in a human matrix.
Occurrence and Concentration of Chemical Additives in Consumer Products in Korea.[Pubmed:31842379]
Int J Environ Res Public Health. 2019 Dec 12;16(24). pii: ijerph16245075.
As the variety of chemicals used in consumer products (CPs) has increased, concerns about human health risk have grown accordingly. Even though restrictive guidelines and regulations have taken place to minimize the risks, human exposure to these chemicals and their eco-compatibility has remained a matter of greater scientific concern over the years. A major challenge in understanding the reality of the exposure is the lack of available information on the increasing number of ingredients and additives in the products. Even when ingredients of CPs formulations are identified on the product containers, the concentrations of the chemicals are rarely known to consumers. In the present study, an integrated target/suspect/non-target screening procedure using liquid chromatography-high resolution mass spectrometry (LC-HRMS) with stepwise identification workflow was used for the identification of known, suspect, and unknown chemicals in CPs including cosmetics, personal care products, and washing agents. The target screening was applied to identify and quantify isothiazolinones and phthalates. Among analyzed CPs, isothiazolinones and phthalates were found in 47% and in 24% of the samples, respectively. The highest concentrations were 518 mg/kg for benzisothiazolone, 7.1 mg/kg for methylisothiazolinone, 2.0 mg/kg for diethyl phthalate, and 21 mg/kg for dimethyl phthalate. Suspect and non-target analyses yielded six tentatively identified chemicals across the products including benzophenone, Ricinine, iodocarb (IPBC), galaxolidone, triethanolamine, and 2-(2H-Benzotriazol-2-yl)-4, 6-bis (1-methyl-1-phenylethyl) phenol. Our results revealed that selected CPs consistently contain chemicals from multiple classes. Excessive use of these chemicals in daily life can increase the risk for human health and the environment.
Ent-trachylobane-3beta-hydroperoxide, a new diterpene from the root bark of Chrozophora oblongifolia (Fam.; Euphorbiaceae).[Pubmed:31691590]
Nat Prod Res. 2019 Nov 6:1-8.
New ent-trachylobane-3beta-hydroperoxide (5) together with four known compounds, Ricinine (1), trimethoxy ellagic acid (2), dimethoxy ellagic acid (3) and aleurotolic acid (4) were isolated from the methylene chloride fraction of the root bark of Chrozophora oblongifolia. The structures of these secondary metabolites were elucidated by using different spectroscopic techniques, (1)H NMR, (13)C NMR, HSQC, HMBC, (1)H-(1)H COSY, NOESY, HR-ESI-MS, EI-MS and comparison with published data. Ent-trachylobane-3beta-hydroperoxide showed moderate cytotoxic activity by MTT assay method against human breast cancer cells (MCF-7) and human hepatocyte-derived carcinoma cells (Huh-7) with IC50 values of 24.53 and 34.13 microM, in comparison with IC50 values of 23.47 microM and 15.82 microM for 5-fluorouracil respectively.
Ricinus communis L. fruit extract inhibits migration/invasion, induces apoptosis in breast cancer cells and arrests tumor progression in vivo.[Pubmed:31601896]
Sci Rep. 2019 Oct 10;9(1):14493.
Medicinal plant-based therapies can be important for treatment of cancer owing to high efficiency, low cost and minimal side effects. Here, we report the anti-cancer efficacy of Ricinus communis L. fruit extract (RCFE) using estrogen positive MCF-7 and highly aggressive, triple negative MDA-MB-231 breast cancer cells. RCFE induced cytotoxicity in these cells in dose and time-dependent manner. It also demonstrated robust anti-metastatic activity as it significantly inhibited migration, adhesion, invasion and expression of matrix metalloproteinases (MMPs) 2 and 9 in both cell lines. Further, flow cytometry analysis suggested RCFE-mediated induction of apoptosis in these cells. This was supported by attenuation of anti-apoptotic Bcl-2, induction of pro-apoptotic Bax and caspase-7 expressions as well as PARP cleavage upon RCFE treatment. RCFE (0.5 mg/Kg body weight) treatment led to significant reduction in tumor volume in 4T1 syngeneic mouse model. HPLC and ESI-MS analysis of active ethyl acetate fraction of RCFE detected four compounds, Ricinine, p-Coumaric acid, Epigallocatechin and Ricinoleic acid. Individually these compounds showed cytotoxic and migration-inhibitory activities. Overall, this study for the first time demonstrates the anti-cancer efficacy of the fruit extract of common castor plant which can be proposed as a potent candidate for the treatment of breast cancer.
Microwave-Assisted Extraction of Ricinine from Ricinus communis Leaves.[Pubmed:31581463]
Antioxidants (Basel). 2019 Oct 1;8(10). pii: antiox8100438.
The alkaloid Ricinine (3-cyano-4-methoxy-N-methyl-2-pyridone) is found in different parts of the Ricinus communis plant and is known to possess several bioactive properties, including strong antioxidant activity. In this study, a new microwave-assisted extraction (MAE) method was developed for the recovery of Ricinine from R. communis leaves. The extraction variables studied were extraction temperature (between 125 degrees C and 175 degrees C), microwave power (between 500 W and 1000 W), extraction time (between 5 min and 15 min), extraction solvent (between 10% and 90% of EtOAc in MeOH), and solvent-to-sample ratio (between 25:1 mL and 50:1 mL of solvent per gram of the sample). On studying the effects of extraction variables, both solvent and liquid-to-solid ratio were found to exhibit the highest effects on Ricinine recovery. A fast (15 min) microwave-assisted extraction method was developed (high temperatures can be applied because the stability of Ricinine is proven in the literature), allowing for the recovery of Ricinine from R. communis leaves. The study revealed that R. communis leaves had almost 1.5 mg g(-1) (dried weight) of Ricinine.
Synthesis of new selective cytotoxic ricinine analogues against oral squamous cell carcinoma.[Pubmed:31526148]
Nat Prod Res. 2021 Jul;35(13):2145-2156.
Sixteen new analogues were synthesized from Ricinine and tested alongside with seven known analogues for their cytotoxic activity against oral cancer (SAS cells) and normal epithelial cells (L132 cells). In contrast to 5-FU, the synthesized Ricinine analogues did not show toxicity to normal cells. However, some of them inhibited the proliferation of oral cancer cells at 25 microM as evident from the MTT assay results. Ricinine analogue (19) was shown to be the most active derivative (69.22% inhibition). Potential targets involved in the oral cancer inhibitory activity of compound 19 were investigated using in-silico studies and western blot analysis. PTP1B was predicted to be a target for Ricinine using reverse docking approach. This prediction was confirmed by western blot analysis that revealed the downregulation of PTP1B protein by compound 19. Moreover, it showed downregulation of COX-2 which is also extensively expressed in oral cancer.
[Simultaneous LC-MS/MS Determination of 18 Plant Toxins in Beverages].[Pubmed:31474651]
Shokuhin Eiseigaku Zasshi. 2019;60(4):108-112.
Assuming the intentional adulteration of beverages with plant toxins, an LC-MS/MS method for the simultaneous determination of 18 plant toxins (lycorine, galantamine, Ricinine, scopolamine, gelsemine, atropine, colchicine, alpha-solanine, jervine, alpha-chaconine, veratramine, mesaconitine, digoxin, protoveratrine A, aconitine, hypaconitine, oleandrin, and digitoxin) was developed. As analytical samples, beer, distilled spirits, blend tea, ready-to-drink (RTD) coffee, and fermented milk drink were selected. The extraction and purification of the analytes were performed using modified QuEChERS method. Method validation in terms of intra-day precision, accuracy, and extraction recovery obtained satisfactory results. The calibration curves for the analytes were linear from 5 to 200 ng/mL (r>0.990), which enabled the determination of toxins in even trace amounts.
Massive fatal overdose of abrin with progressive encephalopathy(.)[Pubmed:31456429]
Clin Toxicol (Phila). 2020 May;58(5):417-420.
Introduction: The jequirity bean (Abrus precatorius) seed contains abrin, a toxalbumin, that irreversibly binds the 60-s ribosomal subunit inhibiting protein synthesis. Neurologic manifestations of ingestions are rare.Case details: We present a case of a 20-year-old man with 24 h of vomiting, diarrhea and 2 h of hematemesis and hematochezia. He admitted to purchasing 1000 jequirity beans online, crushing and ingesting them 26 h prior to presentation in a suicide attempt. Over the next 2 days, he developed hallucinations, incomprehensible mumbling and grunting, disconjugate gaze with abnormal roving eye movements and a left gaze preference with his right eye deviated medially. There was a fine tremor of the upper extremities and he had brief episodes of choreoathetoid movements of his legs. A head CT was normal with no cerebral edema. He progressed to minimally responsive to noxious stimuli, and was unable to converse or follow commands and displayed increased choreoathetoid movements of his extremities. An electroencephalogram (EEG) showed only mild background slowing. Magnetic resonance imaging (MRI) was performed showing bilaterally symmetric signal abnormalities in the basal ganglia, brainstem, corpus callosum and corona radiata with diffuse leptomeningeal enhancement. The patient developed a tonic-clonic seizure followed by pulseless electrical activity, from which he was resuscitated. He was provided comfort care and died just under 5 days after his ingestion.Results: Urine analysis using liquid chromatography coupled to tandem mass spectrometry was positive for 8.84 ng/ml of l-abrine (4.96 ng l-abrine/mg creatinine) 61 h after admission to the hospital (approximately 87 h post-ingestion). Serum concentrations for l-abrine and Ricinine were both below the limits of detection.Discussion: Ingestion of 1000 crushed jequirity beans purchased on the internet resulted in progressive encephalopathy and death.


