Cucurbitacin FCAS# 5939-57-1 |
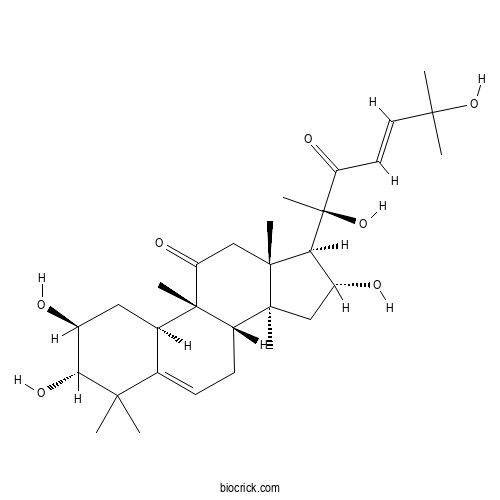
- Cucurbitacin O
Catalog No.:BCN0921
CAS No.:25383-23-7
- Presenegenin
Catalog No.:BCX1141
CAS No.:2163-40-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5939-57-1 | SDF | Download SDF |
| PubChem ID | 5281320 | Appearance | Powder |
| Formula | C30H46O7 | M.Wt | 518.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S,8S,9R,10R,13R,14S,16R,17R)-17-[(E,2R)-2,6-dihydroxy-6-methyl-3-oxohept-4-en-2-yl]-2,3,16-trihydroxy-4,4,9,13,14-pentamethyl-1,2,3,7,8,10,12,15,16,17-decahydrocyclopenta[a]phenanthren-11-one | ||
| SMILES | CC1(C(C(CC2C1=CCC3C2(C(=O)CC4(C3(CC(C4C(C)(C(=O)C=CC(C)(C)O)O)O)C)C)C)O)O)C | ||
| Standard InChIKey | AOHIGMQGPFTKQX-QZPKXHNASA-N | ||
| Standard InChI | InChI=1S/C30H46O7/c1-25(2,36)12-11-21(33)30(8,37)23-19(32)14-27(5)20-10-9-16-17(13-18(31)24(35)26(16,3)4)29(20,7)22(34)15-28(23,27)6/h9,11-12,17-20,23-24,31-32,35-37H,10,13-15H2,1-8H3/b12-11+/t17-,18+,19-,20+,23+,24-,27+,28-,29+,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cucurbitacin F Dilution Calculator

Cucurbitacin F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9279 mL | 9.6395 mL | 19.279 mL | 38.5579 mL | 48.1974 mL |
| 5 mM | 0.3856 mL | 1.9279 mL | 3.8558 mL | 7.7116 mL | 9.6395 mL |
| 10 mM | 0.1928 mL | 0.9639 mL | 1.9279 mL | 3.8558 mL | 4.8197 mL |
| 50 mM | 0.0386 mL | 0.1928 mL | 0.3856 mL | 0.7712 mL | 0.9639 mL |
| 100 mM | 0.0193 mL | 0.0964 mL | 0.1928 mL | 0.3856 mL | 0.482 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cucurbitacin H
Catalog No.:BCN0917
CAS No.:751-95-1
- Cucurbitacin C
Catalog No.:BCN0916
CAS No.:5988-76-1
- Cinnamtannin D2
Catalog No.:BCN0915
CAS No.:97233-47-1
- Cinnamtannin D1
Catalog No.:BCN0914
CAS No.:97233-06-2
- Cinnamtannin B2
Catalog No.:BCN0913
CAS No.:88038-12-4
- Oenothein B
Catalog No.:BCN0912
CAS No.:104987-36-2
- Cinnamtannin A4
Catalog No.:BCN0911
CAS No.:88847-05-6
- 2-O-Acetylzeylenone
Catalog No.:BCN0910
CAS No.:358748-29-5
- Procyanidin B4 3'-O-gallate
Catalog No.:BCN0909
CAS No.:89064-33-5
- Procyanidin B2 3-O-gallate
Catalog No.:BCN0908
CAS No.:109280-47-9
- Procyanidin B1 3-O-gallate
Catalog No.:BCN0907
CAS No.:79907-45-2
- Leucocyanidin
Catalog No.:BCN0906
CAS No.:69256-15-1
- Cucurbitacin L
Catalog No.:BCN0919
CAS No.:1110-02-7
- Cucurbitacin P
Catalog No.:BCN0920
CAS No.:25383-26-0
- Cucurbitacin O
Catalog No.:BCN0921
CAS No.:25383-23-7
- Cucurbitacin K
Catalog No.:BCN0922
CAS No.:6766-43-4
- Cucurbitacin J
Catalog No.:BCN0923
CAS No.:5979-41-9
- 6-hydroxyapigenin-6-O-β-D-glucoside-7-O-β-D-glucuronide
Catalog No.:BCN0924
CAS No.:1146045-40-0
- Cistanoside B
Catalog No.:BCN0925
CAS No.:93236-41-0
- Purpureaside B
Catalog No.:BCN0926
CAS No.:104777-69-7
- Jionoside C
Catalog No.:BCN0927
CAS No.:120406-33-9
- Polygalasaponin B
Catalog No.:BCN0928
CAS No.:103444-92-4
- polygalasaponin Ⅰ
Catalog No.:BCN0929
CAS No.:162901-83-9
- Polygalasaponin XXXII
Catalog No.:BCN0930
CAS No.:176182-04-0
Metabolome and Transcriptome Analyses of Cucurbitacin Biosynthesis in Luffa (Luffa acutangula).[Pubmed:35747880]
Front Plant Sci. 2022 Jun 7;13:886870.
Cucurbitacins are extremely bitter compounds mainly present in Cucurbitaceae, where Luffa belongs. However, there is no comprehensive analysis of cucurbitacin biosynthesis in Luffa fruit. Therefore, this study analyzed bitter (WM709) and non-bitter (S1174) genotypes of Luffa to reveal the underlying mechanism of cucurbitacin biosynthesis by integrating metabolome and transcriptome analyses. A total of 422 metabolites were detected, including vitamins, essential amino acids, antioxidants, and antitumor substances. Of these, 131 metabolites showed significant differences between bitter (WM709) and non-bitter (S1174) Luffa fruits. The levels of isocucurbitacin B, cucurbitacin D, 23,24-dihydro cucurbitacin E, Cucurbitacin F were significantly higher in bitter than in non-bitter Luffa. Transcriptome analysis showed that Bi, cytochromes P450s (CYP450s), and acyltransferase (ACT) of the cucurbitacin biosynthesis pathway, were significantly up-regulated. Moreover, drought stress and abscisic acid (ABA) activated genes of the cucurbitacin biosynthesis pathway. Furthermore, dual-luciferase reporter and yeast one-hybrid assays demonstrated that ABA-response element binding factor 1 (AREB1) binds to the Bi promoter to activate Bi expression. Comparative analysis of the Luffa and cucumber genomes showed that Bi, CYP450s, and ACT are located in the conserved syntenic loci, and formed a cucurbitacin biosynthesis cluster. This study provides important insights into major genes and metabolites of the cucurbitacin biosynthetic pathway, deepening the understanding of regulatory mechanisms of cucurbitacin biosynthesis in Luffa.
[Chemical Compositions from Stems and Branches of Sorbaria arborea].[Pubmed:27254923]
Zhong Yao Cai. 2015 Oct;38(10):2098-101.
OBJECTIVE: To investigate the chemical constituents from the stems and branches of Sorbaria arborea. METHODS: The chemical constituents were isolated and purified by silica gel column chromatography, Sephadex LH-20 column chromatography and recrystallization. Their structures were identified by physicochemical properties and spectra analysis. RESULTS: Ten compounds were isolated and identified as ursolic acid (1), Cucurbitacin F (2), (-) -epicatechin (3), daucosterol (4), arbutin (5), 3-O-beta-anthemisol (6), 2,6-dimethoxy-p-hydroquinone-4-O-beta-D-glucopyranoside (7), lupeol (8), betulin (9) and lup-20 (29) -en-3beta, 30-diol (10). CONCLUSION: All the compounds are isolated from this plant for the first time, and compounds 1, 6 - 8 and 10 are obtained from Sorbaria genus for the first time.
25-O-acetyl-23,24-dihydro-cucurbitacin F induces cell cycle G2/M arrest and apoptosis in human soft tissue sarcoma cells.[Pubmed:25701753]
J Ethnopharmacol. 2015 Apr 22;164:265-72.
ETHNOPHARMACOLOGICAL RELEVANCE: Quisqualis indica is used in traditional Chinese medicine to treat cancer and related syndromes and also known for its anthelminthic effects. AIM OF THE STUDY: Soft tissue sarcomas represent a rare group of malignant tumors that frequently exhibit chemotherapeutic resistance and increased metastatic potential. In this study, we evaluated the cytotoxic, apoptosis inducing and cell cycle arresting effects of 25-O-acetyl-23,24-dihydro-Cucurbitacin F which has been isolated from leaves and twigs of Q. indica. MATERIAL AND METHODS: The present study investigates the effects of 25-O-acetyl-23,24-dihydro-Cucurbitacin F (1) on cell viability, cell cycle distribution, and apoptotic induction of three human sarcoma cell lines of various origins by using the CellTiter 96((R)) AQueous One Solution Cell Proliferation Assay, flow cytometrical experiments, real-time RT-PCR, Western blotting, and the Caspase-Glo((R)) 3/7 Assay RESULTS: We could show that 1 reduced cell viability in a dose-dependent manner and arrested the cells at the G2/M interface. The accumulation of cells at the G2/M phase resulted in a significant decrease of the cell cycle checkpoint regulators cyclin B1, cyclin A, CDK1, and CDK2. Interestingly, 1 inhibited survivin expression significantly, which functions as a key regulator of mitosis and programmed cell death, and is overexpressed in many tumor types including sarcomas. Moreover, 1 induced apoptosis in liposarcoma and rhabdomyosarcoma cells caspase-3 dependently. CONCLUSION: Our data strongly support 1 as a very interesting target for further investigation and development of novel therapeutics in sarcoma research.
Three new cucurbitane triterpenoids from Hemsleya penxianensis and their cytotoxic activities.[Pubmed:24717151]
Bioorg Med Chem Lett. 2014 May 1;24(9):2159-62.
Two new cucurbitane glycosides, hemslepenside A (1) and 16,25-O-diacetyl-Cucurbitacin F-2-O-beta-d-glucopyranoside (3), one new cucurbitacin, 16-O-acetyl-Cucurbitacin F (2), along with three known cucurbitane compounds, were isolated from the roots of Hemsleya penxianensis. The structures of 1-6 were established on the basis of extensive spectroscopic and chemical methods. The isolated compounds were evaluated for their cytotoxic activities against different three human cancer cell lines, with IC50 values in the low microgram range.
[Chemical components in Hemsleya chensnsis (III)].[Pubmed:22715729]
Zhongguo Zhong Yao Za Zhi. 2012 Mar;37(6):814-7.
OBJECTIVE: To study the chemical constituents of Hemsleya chensnsis. METHOD: Various chromatographic techniques were used to separate and purify the constituents. The spectral data (MS, NMR) were obtained for structure elucidation. RESULT: Eight known triterpenoid saponins were isolated from the root of H. chensnsis. Their structures were elucidated as dihydrocucurbitacin B (1), 25-O-acetyl-dihydroCucurbitacin F (2), Cucurbitacin F (3), 3-O-(6'-ethyl ester) -beta-D-glucuropyranosyl oleanolic acid-28-O-alpha-L-arabinopyranoside (4), oleanolic acid-3-(6'-methyl ester) -beta-D-glucuropyranosyl (1-3) -alpha-L-arabinopyranoside (5), oleanolic acid-28-O-beta-D-glucopyranosyl-(1-6) -beta-D-glucopyranoside (6), 3-O-(6'-methyl ester) -beta-D-glucuropyranosyl oleanolic acid-28-O-beta-D-glucopyranosyl-(1-6) -beta-D-glucopyranoside (7), 3-O-(6'-methyl ester) -beta-D-glucuropyranosyl (1-2) -beta-D-glucopyranoside-oleanolic acid-28-O-beta-D-glucopyranoside (8). CONCLUSION: All compounds except for 2 were isolated from H. chensnsis for the first time.
Actin-aggregating cucurbitacins from Physocarpus capitatus.[Pubmed:18959442]
J Nat Prod. 2008 Nov;71(11):1927-9.
Bioassay-guided fractionation of Physocarpus capitatus yielded two new cucurbitacins (3 and 4) along with the known Cucurbitacin F (1) and dihydroCucurbitacin F (2). Preliminary mechanism of action studies indicate that the cucurbitacins cause actin aggregates and inhibit cell division.
Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese materia medica with activity against tumor cells.[Pubmed:18202018]
Mol Cancer Ther. 2008 Jan;7(1):152-61.
The cure from cancer is still not a reality for all patients, which is mainly due to the limitations of chemotherapy (e.g., drug resistance and toxicity). Apart from the high-throughput screening of synthetic chemical libraries, natural products represent attractive alternatives for drug development. We have done a systematic bioactivity-based screening of natural products derived from medicinal plants used in traditional Chinese medicine. Plant extracts with growth-inhibitory activity against tumor cells have been fractionated by chromatographic techniques. We have isolated the bioactive compounds and elucidated the chemical structures by nuclear magnetic resonance and mass spectrometry. By this strategy, we identified 25-O-acetyl-23,24-dihydro-Cucurbitacin F as a cytotoxic constituent of Quisqualis indica. Another promising compound identified by this approach was miltirone from Salvia miltiorrhiza. The IC50 values for miltirone of 60 National Cancer Institute cell lines were associated with the microarray-based expression of 9,706 genes. By COMPARE and hierarchical cluster analyses, candidate genes were identified, which significantly predicted sensitivity or resistance of cell lines to miltirone.
Ellagic acid derivatives and cytotoxic cucurbitacins from Elaeocarpus mastersii.[Pubmed:12169311]
Phytochemistry. 2002 Sep;61(2):171-4.
Bioassay-guided investigation of the bark of Elaeocarpus mastersii using KB (human oral epidermoid carcinoma) cells as a monitor led to the isolation of two cucurbitacins, cucurbitacin D and Cucurbitacin F as cytotoxic principles, together with two ellagic acid derivatives, 4'-O-methylellagic acid 3-(2",3"-di-O-acetyl)-alpha-L-rhamnoside (1) and 4,4'-O-dimethylellagic acid 3-(2",3"-di-O-acetyl)-alpha-L-rhamnoside (2). These compounds were evaluated against a panel of human tumor cell lines.
Cucurbitacin F in seeds of Kageneckia angustifolia (Rosaceae).[Pubmed:11926537]
Z Naturforsch C J Biosci. 2002 Jan-Feb;57(1-2):208-9.
Cucurbitacin F and the cyanogenetic compounds prunasin were isolated and identified from the seeds of Kageneckia angustifolia.
Pharmaco-toxicological study of Kageneckia oblonga, Rosaceae.[Pubmed:11926521]
Z Naturforsch C J Biosci. 2002 Jan-Feb;57(1-2):100-8.
The probable antipyretic, antiinflammatory, analgesic and antioxidant properties of Kageneckia oblonga, Rosaceae, were investigated and the major compounds of its active extracts were isolated. The study comprised the acute toxicity of the extracts of global methanol, hexane, dichloromethane and methanol. The cytotoxicity of global methanol extract was studied in three tumoral cell lines. All the extracts exhibited the pharmacological activities under study. Methanol and dichloromethane were the most toxic extracts. From the global methanol extract, isolations were performed of prunasin, 23,24- dihydro-Cucurbitacin F, and a new cucurbitacin, 3beta-(beta-D-glucosyloxy)-16alpha,23alpha-epoxycucurbita-5,24-diene-11-one. The cytotoxicity of both cucurbitacins on human neutrophils at the assayed concentrations was not statistically significant. In-vitro assays showed that both cucurbitacins can be partly responsible for the analgesic, antipyretic, and anti-inflammatory activities. Evaluation was done of the cytotoxicity of global methanol extract, 23, 24-dihydroCucurbitacin F, aqueous extracts and prunasin against P-388 murine leukaemia, A-549 human lung carcinoma and HT-29 colon carcinoma. Since global methanol extract presented a strong cytotoxicity against P-388 murine leukaemia, A-549 human lung carcinoma, and HT-29 cell lines, it is highly probable that this extract contain one or more cytotoxic compounds that could be investigated for their potential use as an agent against cancer.
[Chemical constituents of Hemsleya graciliflora (Harms) Cogn].[Pubmed:11038887]
Zhongguo Zhong Yao Za Zhi. 1997 Jun;22(6):357-8, 384.
Nine compounds were isolated from the fruits of Hemsleya graciliflora. IIThey were identified as- 25-acetate of dihydroCucurbitacin F(I), dihydroCucurbitacin F(II), Cucurbitacin F-25-acetate(III), sitostery1-3-O-beta-D-glucoside-6'-O-palmitate(IV), daucosterol(V), beta-sitosterol(VI), palmitic acid(VII), octodecyl acohol(VIII) and hexadecanol(IX) on the basis of physical and chemical properties as well as spectral data. The compound(IV) is isolated from genus Hemsleya for the first time.
Cytotoxic constituents ofSorbaria sorbifolia var.stellipila.[Pubmed:18975218]
Arch Pharm Res. 1997 Feb;20(1):85-7.
The acivity-guided fractionation upon the MeOH extract of the aerial parts ofSorbaria sorbifolia var.stellipila led to the isolation of two cucurbitacin-compounds, cucurbitacin D and Cucurbitacin F, as active principles. Two compounds were shown to exhibit significant cytotoxicity against cultured human tumor cell lines, A-549, SK-OV-3, SK-MEL-2, XF-498, and HCT 15.
Inhibitory effects of cucurbitane triterpenoids on Epstein-Barr virus activation and two-stage carcinogenesis of skin tumors.[Pubmed:7920430]
Biol Pharm Bull. 1994 May;17(5):668-71.
To search for possible anti-tumor-promoters, we carried out a primary screening of 21 cucurbitane triterpenoids using an in vitro assay system. Of these triterpenoids, scandenoside R6 (6), 23,24-dihydroCucurbitacin F (14), 25-acetyl-23,24-dihydroCucurbitacin F (15), 2-O-beta-D-glucopyranosyl-23,24-dihydroCucurbitacin F (17) and Cucurbitacin F (18) exhibited significant inhibitory effects on Epstein-Barr virus (EBV) activation induced by the tumor promoter, 12-O-tetradecanoyl-phorbol-13-acetate (TPA). Further, compounds 14 and 17 exhibited remarkable anti-tumor-promotion effects on mouse skin tumor promotion in an in vivo two-stage carcinogenesis test.
Constituents of rosaceous plants. I. Structure of new triterpenoids from Cowania mexicana.[Pubmed:8221976]
Chem Pharm Bull (Tokyo). 1993 Sep;41(9):1612-5.
In our search for possible anti-tumor-promoters, we carried out an investigation of the leaves and branches of Cowania mexicana D. DON (Rosaceae). Two new cucurbitane type triterpenes, 15-oxo-Cucurbitacin F (3) and 15-oxo-23,24-dihydroCucurbitacin F (4), were isolated together with Cucurbitacin F (1) and 23,24-dihydroCucurbitacin F (2). These triterpenes were inhibitorss of Epstein-Barr virus early antigen activation induced by 12-O-tetradecanoylphorbol-13-acetate, a well-known tumor-promoter. The structures of 3 and 4 were determined from 2D-NMR spectral data and difference NOE experiments.


