LeucocyanidinCAS# 69256-15-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

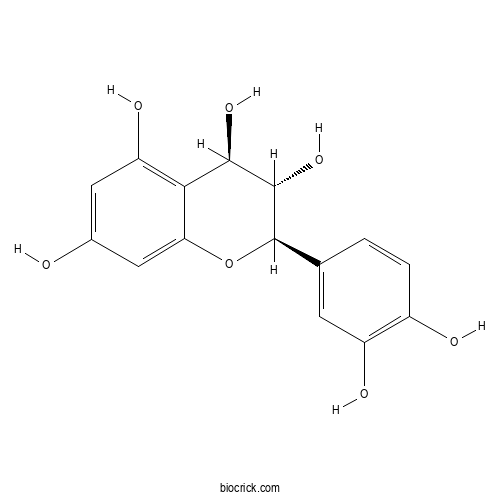
| Cas No. | 69256-15-1 | SDF | Download SDF |
| PubChem ID | 155206 | Appearance | Powder |
| Formula | C15H14O7 | M.Wt | 306.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S,4R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,4,5,7-tetrol | ||
| SMILES | C1=CC(=C(C=C1C2C(C(C3=C(C=C(C=C3O2)O)O)O)O)O)O | ||
| Standard InChIKey | SBZWTSHAFILOTE-QLFBSQMISA-N | ||
| Standard InChI | InChI=1S/C15H14O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,13-21H/t13-,14+,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Leucocyanidin Dilution Calculator

Leucocyanidin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2648 mL | 16.3239 mL | 32.6477 mL | 65.2955 mL | 81.6193 mL |
| 5 mM | 0.653 mL | 3.2648 mL | 6.5295 mL | 13.0591 mL | 16.3239 mL |
| 10 mM | 0.3265 mL | 1.6324 mL | 3.2648 mL | 6.5295 mL | 8.1619 mL |
| 50 mM | 0.0653 mL | 0.3265 mL | 0.653 mL | 1.3059 mL | 1.6324 mL |
| 100 mM | 0.0326 mL | 0.1632 mL | 0.3265 mL | 0.653 mL | 0.8162 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Peanut procyanidin F
Catalog No.:BCN0905
CAS No.:2095621-25-1
- Peanut procyanidin E
Catalog No.:BCN0904
CAS No.:2095621-10-4
- Peanut procyanidin D
Catalog No.:BCN0903
CAS No.:114637-81-9
- Peanut procyanidin C
Catalog No.:BCN0902
CAS No.:2095621-31-9
- Peanut procyanidin B
Catalog No.:BCN0901
CAS No.:2085301-50-2
- Peanut procyanidin A
Catalog No.:BCN0900
CAS No.:1802175-67-2
- Cinnamtannin B1
Catalog No.:BCN0899
CAS No.:88082-60-4
- Procyanidin C2
Catalog No.:BCN0898
CAS No.:37064-31-6
- Procyanidin B8
Catalog No.:BCN0897
CAS No.:12798-60-6
- Procyanidin B7
Catalog No.:BCN0896
CAS No.:12798-59-3
- Procyanidin B6
Catalog No.:BCN0895
CAS No.:12798-58-2
- Procyanidin A7
Catalog No.:BCN0894
CAS No.:114569-32-3
- Procyanidin B1 3-O-gallate
Catalog No.:BCN0907
CAS No.:79907-45-2
- Procyanidin B2 3-O-gallate
Catalog No.:BCN0908
CAS No.:109280-47-9
- Procyanidin B4 3'-O-gallate
Catalog No.:BCN0909
CAS No.:89064-33-5
- 2-O-Acetylzeylenone
Catalog No.:BCN0910
CAS No.:358748-29-5
- Cinnamtannin A4
Catalog No.:BCN0911
CAS No.:88847-05-6
- Oenothein B
Catalog No.:BCN0912
CAS No.:104987-36-2
- Cinnamtannin B2
Catalog No.:BCN0913
CAS No.:88038-12-4
- Cinnamtannin D1
Catalog No.:BCN0914
CAS No.:97233-06-2
- Cinnamtannin D2
Catalog No.:BCN0915
CAS No.:97233-47-1
- Cucurbitacin C
Catalog No.:BCN0916
CAS No.:5988-76-1
- Cucurbitacin H
Catalog No.:BCN0917
CAS No.:751-95-1
- Cucurbitacin F
Catalog No.:BCN0918
CAS No.:5939-57-1
A role for ascorbate conjugates of (+)-catechin in proanthocyanidin polymerization.[Pubmed:35701431]
Nat Commun. 2022 Jun 14;13(1):3425.
Proanthocyanidins (PAs) are natural polymers of flavan-3-ols, commonly (+)-catechin and (-)-epicatechin. However, exactly how PA oligomerization proceeds is poorly understood. Here we show, both biochemically and genetically, that ascorbate (AsA) is an alternative "starter unit" to flavan-3-ol monomers for Leucocyanidin-derived (+)-catechin subunit extension in the Arabidopsis thaliana anthocyanidin synthase (ans) mutant. These (catechin)n:ascorbate conjugates (AsA-[C]n) also accumulate throughout the phase of active PA biosynthesis in wild-type grape flowers, berry skins and seeds. In the presence of (-)-epicatechin, AsA-[C]n can further provide monomeric or oligomeric PA extension units for non-enzymatic polymerization in vitro, and their role in vivo is inferred from analysis of relative metabolite levels in both Arabidopsis and grape. Our findings advance the knowledge of (+)-catechin-type PA extension and indicate that PA oligomerization does not necessarily proceed by sequential addition of a single extension unit. AsA-[C]n defines a new type of PA intermediate which we term "sub-PAs".
Dual activity of anthocyanidin reductase supports the dominant plant proanthocyanidin extension unit pathway.[Pubmed:33990337]
Sci Adv. 2021 May 14;7(20). pii: 7/20/eabg4682.
Proanthocyanidins (PAs) are plant natural products important for agriculture and human health. They are polymers of flavan-3-ol subunits, commonly (-)-epicatechin and/or (+)-catechin, but the source of the in planta extension unit that comprises the bulk of the polymer remains unclear, as does how PA composition is determined in different plant species. Anthocyanidin reductase (ANR) can generate 2,3-cis-epicatechin as a PA starter unit from cyanidin, which itself arises from 2,3-trans-Leucocyanidin, but ANR proteins from different species produce mixtures of flavan-3-ols with different stereochemistries in vitro. Genetic and biochemical analyses here show that ANR has dual activity and is involved not only in the production of (-)-epicatechin starter units but also in the formation of 2,3-cis-Leucocyanidin to serve as (-)-epicatechin extension units. Differences in the product specificities of ANRs account for the presence/absence of PA polymerization and the compositions of PAs across plant species.
Comprehensive Analysis of Metabolic Fluxes from Leucoanthocyanins to Anthocyanins and Proanthocyanidins (PAs).[Pubmed:33307696]
J Agric Food Chem. 2020 Dec 23;68(51):15142-15153.
Anthocyanins and PAs are the two most common flavonoids, which are widely present among diverse species. Great progress has been made in their synthesis and regulation. In this study, we analyzed the metabolic fluxes from their synthetic precursor leucoanthocyanins, which were obtained by overexpression of dihydroflavonol 4-reductase (DFR) in vitro and in vivo. The unstable product Leucocyanidin generated in the CsDFRa enzymatic reaction was easily converted into C-type carbocations under weak acidic conditions, which could be further involved in the synthesis of C-type PAs in vitro. Additionally, the metabolites in tobacco overexpressing CsDFRa and Arabidopsis thaliana DFR and anthocyanidin synthase (ANS) mutants were investigated. In CsDFRa transgenic tobacco, the content of anthocyanins in the petals was greatly increased, but no catechin or PA was detected. In A. thaliana, EC-type carbocation was mainly accumulated in the wild type (WT), and the C-type carbocation was only detected in the ans mutant. In tea plant, the accumulation of C-type PAs is strong positively correlated with the expression of CsDFRa. In summary, Leucocyanidin is not only involved in the synthesis of downstream anthocyanin and epicatechin but also can be converted into C-type carbocation to participate in the synthesis of C-type PAs. Hence, from Leucocyanidin, three metabolic fluxes were formed toward catechin, cyanidin, and C-type carbocation. These results enriched the metabolic fluxes of leucoanthocyanins and further elaborated the roles of DFR in the process of C-type PA formation.
Metabolic Flux Analysis of Catechin Biosynthesis Pathways Using Nanosensor.[Pubmed:32244268]
Antioxidants (Basel). 2020 Mar 31;9(4). pii: antiox9040288.
(+)-Catechin is an important antioxidant of green tea (Camelia sinensis (L.) O. Kuntze). Catechin is known for its positive role in anticancerous activity, extracellular matrix degradation, cell death regulation, diabetes, and other related disorders. As a result of enormous interest in and great demand for catechin, its biosynthesis using metabolic engineering has become the subject of concentrated research with the aim of enhancing (+)-catechin production. Metabolic flux is an essential concept in the practice of metabolic engineering as it helps in the identification of the regulatory element of a biosynthetic pathway. In the present study, an attempt was made to analyze the metabolic flux of the (+)-catechin biosynthesis pathway in order to decipher the regulatory element of this pathway. Firstly, a genetically encoded fluorescence resonance energy transfer (FRET)-based nanosensor (FLIP-Cat, fluorescence indicator protein for (+)-catechin) was developed for real-time monitoring of (+)-catechin flux. In vitro characterization of the purified protein of the nanosensor showed that the nanosensor was pH stable and (+)-catechin specific. Its calculated Kd was 139 microM. The nanosensor also performed real-time monitoring of (+)-catechin in bacterial cells. In the second step of this study, an entire (+)-catechin biosynthesis pathway was constructed and expressed in E. coli in two sets of plasmid constructs: pET26b-PT7-rbs-PAL-PT7-rbs-4CL-PT7-rbs-CHS-PT7-rbs-CHI and pET26b-T7-rbs-F3H-PT7-rbs- DFR-PT7-rbs-LCR. The E. coli harboring the FLIP-Cat was transformed with these plasmid constructs. The metabolic flux analysis of (+)-catechin was carried out using the FLIP-Cat. The FLIP-Cat successfully monitored the flux of catechin after adding tyrosine, 4-coumaric acid, 4-coumaroyl CoA, naringenin chalcone, naringenin, dihydroquercetin, and Leucocyanidin, individually, with the bacterial cells expressing the nanosensor as well as the genes of the (+)-catechin biosynthesis pathway. Dihydroflavonol reductase (DFR) was identified as the main regulatory element of the (+)-catechin biosynthesis pathway. Information about this regulatory element of the (+)-catechin biosynthesis pathway can be used for manipulating the (+)-catechin biosynthesis pathway using a metabolic engineering approach to enhance production of (+)-catechin.
Exploration of the Substrate Diversity of Leucoanthocyanidin Reductases.[Pubmed:32141742]
J Agric Food Chem. 2020 Apr 1;68(13):3903-3911.
Proanthocyanidins (PAs) are mainly composed of epicatechin (EC) or catechin (C) subunits. C-type catechins (C and GC) are generally considered to be catalyzed by Leucocyanidin reductase (LAR). In this study, we re-evaluated the function of LAR. LcLAR1 was isolated from Lotus corniculatus, which is rich in C-type catechins. Overexpression of LcLAR1 in tobacco resulted in a significantly increased content of EC and EC-glucoside. Overexpression of LcLAR1 in Arabidopsis thaliana promoted the accumulation of soluble PAs, including EC, PA dimers, and PA trimers. However, in the transgenic ans mutant overexpressing LcLAR1, the contents of C and C-glucoside were increased. In addition, overexpression of LcLAR1 in L. corniculatus resulted in a significant increase of C levels. Taken together, the products of LcLAR1 depended on the substrates, which revealed the substrate diversity of LcLAR1. Our study provides new insights into the flavonoid pathway, especially the role of LAR.
VvLAR1 and VvLAR2 Are Bifunctional Enzymes for Proanthocyanidin Biosynthesis in Grapevine.[Pubmed:31092697]
Plant Physiol. 2019 Jul;180(3):1362-1374.
Proanthocyanidins (PAs) in grapevine (Vitis vinifera) are found mainly in berries, and their content and degree of polymerization are important for the mouth feel of red wine. However, the mechanism of PA polymerization in grapevine remains unclear. Previous studies in the model legume Medicago truncatula showed that 4beta-(S-cysteinyl)-epicatechin (Cys-EC) is an epicatechin-type extension unit for nonenzymatic PA polymerization, and that leucoanthocyanidin reductase (LAR) converts Cys-EC into epicatechin starter unit to control PA extension. Grapevine possesses two LAR genes, but their functions are not clear. Here, we show that both Cys-EC and 4beta-(S-cysteinyl)-catechin (Cys-C) are present in grapevine. Recombinant VvLAR1 and VvLAR2 convert Cys-C and Cys-EC into (+)-catechin and (-)-epicatechin, respectively, in vitro. The kinetic parameters of VvLARs are similar, with both enzymes being more efficient with Cys-C than with Cys-EC, the 2,3-cis conformation of which results in steric hindrance in the active site. Both VvLARs also produce (+)-catechin from Leucocyanidin, and an inactive VvLAR2 allele reported previously is the result of a single amino acid mutation in the N terminus critical for all NADPH-dependent activities of the enzyme. VvLAR1 or VvLAR2 complement the M. truncatula lar:ldox double mutant that also lacks the leucoanthocyanidin dioxygenase (LDOX) required for epicatechin starter unit formation, resulting in increased soluble PA levels, decreased insoluble PA levels, and reduced levels of Cys-C and Cys-EC when compared to the double mutant, and the appearance of catechin, epicatechin, and PA dimers characteristic of the ldox single mutant in young pods. These data advance our knowledge of PA building blocks and LAR function and provide targets for grapevine breeding to alter PA composition.
Oxidative Transformation of Leucocyanidin by Anthocyanidin Synthase from Vitis vinifera Leads Only to Quercetin.[Pubmed:30865451]
J Agric Food Chem. 2019 Apr 3;67(13):3595-3604.
Anthocyanidin synthase from Vitis vinifera ( VvANS) catalyzes the in vitro transformation of the natural isomer of Leucocyanidin, 2 R,3 S,4 S- cis-Leucocyanidin, into 2 R,4 S-flavan-3,3,4-triol ([M + H](+), m/ z 323) and quercetin. The C3-hydroxylation product 2 R,4 S-flavan-3,3,4-triol is first produced and its C3,C4-dehydration product is in tautomeric equilibrium with (+)-dihydroquercetin. The latter undergoes a second VvANS-catalyzed C3-hydroxylation leading to a 4-keto-2 R-flavan-3,3-gem-diol which upon dehydration gives quercetin. The unnatural isomer of Leucocyanidin, 2 R,3 S,4 R- trans-Leucocyanidin, is similarly transformed into quercetin upon C3,C4-dehydration, but unlike 3,4- cis-Leucocyanidin, it also undergoes some C2,C3-dehydration followed by an acid-catalyzed hydroxyl group extrusion at C4 to give traces of cyanidin. Overall, the C3,C4- trans isomer of Leucocyanidin is transformed into 2 R,4 R-flavan-3,3,4-triol (M + 1, m/ z 323), (+)-DHQ, (-)-epiDHQ, quercetin, and traces of cyanidin. Our data bring the first direct observation of 3,4- cis-Leucocyanidin- and 3,4- trans-Leucocyanidin-derived 3,3-gem-diols, supporting the idea that the generic function of ANS is to catalyze the C3-hydroxylation of its substrates. No cyanidin is produced with the natural cis isomer of Leucocyanidin, and only traces with the unnatural trans isomer, which suggests that anthocyanidin synthase requires other substrate(s) for the in vivo formation of anthocyanidins.
[Influence of light on gene expression of key synthesis enzyme genes FtANR and FtLAR about proanthocyanidin in seeds of homologous plant of food and medicine Fagopyrum tataricum].[Pubmed:29600610]
Zhongguo Zhong Yao Za Zhi. 2018 Feb;43(3):469-477.
Tartary buckwheat Fagopyrum tataricum is an important medicinal and functional herb due to its rich content of flavonoids in the seeds. F.tataricum exhibited good functions for free radicals scavenging anti-oxidation anti-aging activities. Although much genetic knowledge of the synthesis regulation accumulation of rutin the genetic basis of proanthocyanidins(PAs) in tartary buckwheat and their related gene expression changes under different lights(blue red far red ultraviolet light) remain largely unexplored. In this study we cloned one anthocyanidin reductase gene(ANR) and two Leucocyanidin reductase gene(LAR) named FtANRFtLAR1FtLAR3 involved in formation of(+)-catechin and(-)-epicatechin precusor proanthocyanidin by digging out F. tataricum seed transcriptome data. The expression data showed that the opposite influence of red light on these gene transcript level compared to others lights. The expression levels of FtANR and FtLAR1 decreased and FtLAR3 appeared increment after exposed in the red light while the expression levels of those genes appeared opposite result after exposed in the blue and far red light.
Proanthocyanidin Synthesis in Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Fruits.[Pubmed:29541082]
Front Plant Sci. 2018 Feb 28;9:212.
Proanthocyanidins (PAs) are distributed widely in Chinese bayberry fruit and have been associated with human health benefits, but molecular and biochemical characterization of PA biosynthesis remains unclear. Here, two genes encoding key PA biosynthetic enzymes, anthocyanidin reductase (ANR) and leucoanthocyanidin reductase (LAR) were isolated in bayberry fruit. MrANR was highly expressed at the early stage of fruit development when soluble PAs accumulated at high levels. Meanwhile, the transcript abundance of both MrANR and MrLAR observed at the late stage was paralleled with the high amounts of insoluble PAs. LC-MS/MS showed that PAs in developing Chinese bayberry fruits were comprised predominantly of epigallocatechin-3-O-gallate terminal subunits, while the extension subunits were a mixture of epigallocatechin-3-O-gallate, epigallocatechin and catechin. Recombinant MrANR protein converted cyanidin to a mixture of epicatechin and catechin, and delphinidin to a mixture of epigallocatechin and gallocatechin in vitro. Recombinant MrLAR was active with Leucocyanidin as substrate to produce catechin. Ectopic expression of MrANR in tobacco reduced anthocyanin levels but increased PA accumulation. The catechin and epicatechin contents in transgenic flowers overexpressed MrANR were significantly higher than those of wild-type. However, overexpression of MrLAR in tobacco led to an increase in catechin levels but had no impact on PA contents. Quantitative real time PCR revealed that the loss of anthocyanin in transgenic flowers overexpressed MrANR or MrLAR is probably attributed to decreased expression of tobacco chalcone isomerase (CHI) gene. Our results not only reveal in vivo and in vitro functions for ANR and LAR but also provide a resource for understanding the mechanism of PA biosynthesis in Chinese bayberry fruit.
Production of 3,4-cis- and 3,4-trans-Leucocyanidin and Their Distinct MS/MS Fragmentation Patterns.[Pubmed:29231723]
J Agric Food Chem. 2018 Jan 10;66(1):351-358.
(+)-2,3-trans-3,4-cis-Leucocyanidin was produced by acidic epimerization of (+)-2,3-trans-3,4-trans-Leucocyanidin synthesized by reduction of (+)-dihydroquercetin with NaBH4, and structures of the two stereoisomers purified by C18- and phenyl-reverse-phase high-performance liquid chromatography (HPLC) were confirmed by NMR spectroscopy. We confirm that only 3,4-cis-Leucocyanidin is used by leucoanthocyanidin reductase as substrate. The two stereoisomers are quite stable in aqueous solution at -20 degrees C. Characterization of the two stereoisomers was also performed using electrospray ionization tandem mass spectrometry (ESI-MS/MS), and we discuss here for the first time the corresponding MS/MS fragmentation pathways, which are clearly distinct. The main difference is that of the mode of dehydration of the 3,4-diol in positive ionization mode, which involves a loss of hydroxyl group at either C3 or C4 for the 3,4-cis isomer but only at C3 for the 3,4-trans isomer. Tandem mass spectrometry therefore proves useful as a complementary methodology to NMR to identify each of the two stereoisomers.
Terminalia catappa: Chemical composition, in vitro and in vivo effects on Haemonchus contortus.[Pubmed:28969774]
Vet Parasitol. 2017 Nov 15;246:118-123.
Haemonchus contortus is the most important nematode in small ruminant systems, and has developed tolerance to all commercial anthelmintics in several countries. In vitro (egg hatch assay) and in vivo tests were performed with a multidrug strain of Haemonchus contortus using Terminalia catappa leaf, fruit pulp, and seed extracts (in vitro), or pulp and seed powder in lambs experimentally infected with H. contortus. Crude extracts from leaves, fruit pulp and seeds obtained with 70% acetone were lyophilized until used. In vitro, the extracts had LC50=2.48mug/mL (seeds), LC50=4.62mug/mL (pulp), and LC50=20mug/mL (leaves). In vitro, seed and pulp extracts had LC50 similar to Thiabendazole (LC50=1.31mug/mL). Condensed tannins were more concentrated in pulp extract (183.92g of Leucocyanidin/kg dry matter) than in either leaf (4.6g) or seed (35.13g) extracts. Phytochemical tests established that all extracts contained alkaloids, flavonoids, saponins, phenols, and terpenoids. Based on these results, in vivo tests were performed to evaluate the anthelmintic activity of T. catappa whole fruit (pulp+seed) powder. Male Santa Ines lambs were artificially infected with multidrug-resistant H. contortus and divided, according to similar fecal egg count (FEC) and weight, into two groups: Control (infected/untreated) and treated (infected/treated with whole fruit powder). Whole fruit powder was mixed with concentrate and provided at 2g/kg of body weight (BW) for five days. After treatment, parasitological analysis (FEC and egg hatch assay), renal profile (urea and creatinine), liver profile (aspartate aminotransferase) and BW were determined. In vitro (based on LC50), seed/pulp extracts had ovicidal effect similar to Thiabendazole but whole fruit powder had no anthelmintic effect on adult nematodes in the abomasum. We discuss the plausible causes of the lack of in vivo activity.
Molecular docking analysis of UniProtKB nitrate reductase enzyme with known natural flavonoids.[Pubmed:28405127]
Bioinformation. 2016 Dec 27;12(12):425-429.
The functional inference of UniProtKB nitrate reductase enzyme (UniProtKB - P0AF33) through structural modeling is of interest in plant biology. Therefore, a homology model for UniProtKB variant of the enzyme was constructed using available data with the MODELER software tool. The model was further docked with five natural flavonoid structures such as hesperetin, naringenin, Leucocyanidin, quercetin and hesperetin triacetate using the AUTODOCK (version 4.2) software tool. The structure aided molecular interactions of these flavonoids with nitrate reductase is documented in this study. The binding features (binding energy (DeltaG) value, H bonds and docking score) hesperetin to the enzyme model is relatively high, satisfactory and notable. This data provides valuable insights to the relative binding of several naturally occurring flavonoids to nitrate reductase enzyme and its relevance in plant biology.
Dihydroflavonol 4-Reductase Genes from Freesia hybrida Play Important and Partially Overlapping Roles in the Biosynthesis of Flavonoids.[Pubmed:28400785]
Front Plant Sci. 2017 Mar 28;8:428.
Dihydroflavonol-4-reductase (DFR) is a key enzyme in the reduction of dihydroflavonols to leucoanthocyanidins in both anthocyanin biosynthesis and proanthocyanidin accumulation. In many plant species, it is encoded by a gene family, however, how the different copies evolve either to function in different tissues or at different times or to specialize in the use of different but related substrates needs to be further investigated, especially in monocot plants. In this study, a total of eight putative DFR-like genes were firstly cloned from Freesia hybrida. Phylogenetic analysis showed that they were classified into different branches, and FhDFR1, FhDFR2, and FhDFR3 were clustered into DFR subgroup, whereas others fell into the group with cinnamoyl-CoA reductase (CCR) proteins. Then, the functions of the three FhDFR genes were further characterized. Different spatio-temporal transcription patterns and levels were observed, indicating that the duplicated FhDFR genes might function divergently. After introducing them into Arabidopsis dfr (tt3-1) mutant plants, partial complementation of the loss of cyanidin derivative synthesis was observed, implying that FhDFRs could convert dihydroquercetin to Leucocyanidin in planta. Biochemical assays also showed that FhDFR1, FhDFR2, and FhDFR3 could utilize dihydromyricetin to generate leucodelphinidin, while FhDFR2 could also catalyze the formation of Leucocyanidin from dihydrocyanidin. On the contrary, neither transgenic nor biochemical analysis demonstrated that FhDFR proteins could reduce dihydrokaempferol to leucopelargonidin. These results were consistent with the freesia flower anthocyanin profiles, among which delphinidin derivatives were predominant, with minor quantities of cyanidin derivatives and undetectable pelargonidin derivatives. Thus, it can be deduced that substrate specificities of DFRs were the determinant for the categories of anthocyanins aglycons accumulated in F. hybrida. Furthermore, we also found that the divergence of the expression patterns for FhDFR genes might be controlled at transcriptional level, as the expression of FhDFR1/FhDFR2 and FhDFR3 was controlled by a potential MBW regulatory complex with different activation efficiencies. Therefore, it can be concluded that the DFR-like genes from F. hybrida have diverged during evolution to play partially overlapping roles in the flavonoid biosynthesis, and the results will contribute to the study of evolution of DFR gene families in angiosperms, especially for monocot plants.
A role for leucoanthocyanidin reductase in the extension of proanthocyanidins.[Pubmed:27869786]
Nat Plants. 2016 Nov 21;2:16182.
Proanthocyanidins (PAs) are the second most abundant plant polyphenolic compounds after lignin. PAs affect taste, mouth feel and astringency of many fruits, wines and beverages(1,2), have been associated with reduced risks of cardiovascular disease, cancer and Alzheimer's disease(3-5), can improve nutrition and prevent bloat in ruminant animals(6) and enhance soil nitrogen retention(7). PAs are oligomers and polymers of flavan-3-ols, primarily (-)-epicatechin and (+)-catechin, but the mechanism by which the monomers polymerize and become insoluble is currently unknown. Leucoanthocyanidin reductase (LAR) has been shown to convert Leucocyanidin to (+)-catechin(8,9). Here, we report that loss of function of LAR in the model legume Medicago truncatula leads unexpectedly to loss of soluble epicatechin-derived PAs, increased levels of insoluble PAs, and accumulation of 4beta-(S-cysteinyl)-epicatechin, which provides the 4-->8 linked extension units during non-enzymatic PA polymerization. LAR converts 4beta-(S-cysteinyl)-epicatechin back to epicatechin, the starter unit in PAs, thereby regulating the relative proportions of starter and extension units and consequently the degree of PA oligomerization.
Enzymatic activity of cell-free extracts from Burkholderia oxyphila OX-01 bio-converts (+)-catechin and (-)-epicatechin to (+)-taxifolin.[Pubmed:27685324]
Biosci Biotechnol Biochem. 2016 Dec;80(12):2473-2479.
This study characterized the enzymatic ability of a cell-free extract from an acidophilic (+)-catechin degrader Burkholderia oxyphila (OX-01). The crude OX-01 extracts were able to transform (+)-catechin and (-)-epicatechin into (+)-taxifolin via a Leucocyanidin intermediate in a two-step oxidation. Enzymatic oxidation at the C-4 position was carried out anaerobically using H2O as an oxygen donor. The C-4 oxidation occurred only in the presence of the 2R-catechin stereoisomer, with the C-3 stereoisomer not affecting the reaction. These results suggest that the OX-01 may have evolved to target both (+)-catechin and (-)-epicatechin, which are major structural units in plants.


