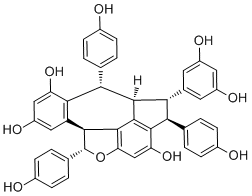
Davidiol ACAS# 881013-34-9 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 881013-34-9 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C42H32O9 | M.Wt | 680.74 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Davidiol A Dilution Calculator

Davidiol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.469 mL | 7.3449 mL | 14.6899 mL | 29.3798 mL | 36.7247 mL |
| 5 mM | 0.2938 mL | 1.469 mL | 2.938 mL | 5.876 mL | 7.3449 mL |
| 10 mM | 0.1469 mL | 0.7345 mL | 1.469 mL | 2.938 mL | 3.6725 mL |
| 50 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5876 mL | 0.7345 mL |
| 100 mM | 0.0147 mL | 0.0734 mL | 0.1469 mL | 0.2938 mL | 0.3672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ethyl (10E,12E)-9-oxooctadeca-10,12-dienoate
Catalog No.:BCX0313
CAS No.:2552582-20-2
- Methyl 2,4-dihydroxybenzoate
Catalog No.:BCX0312
CAS No.:2150-47-2
- 16α-O-Ethylneoquassin
Catalog No.:BCX0311
CAS No.:909272-59-9
- 16β-O-Ethylneoquassin
Catalog No.:BCX0310
CAS No.:343943-63-5
- 16α-O-Methylneoquassin
Catalog No.:BCX0309
CAS No.:89498-94-2
- Curzeone
Catalog No.:BCX0308
CAS No.:104068-56-6
- Kushenol O
Catalog No.:BCX0307
CAS No.:102390-91-0
- Formononetin 7-O-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside
Catalog No.:BCX0306
CAS No.:857677-78-2
- (1S,16R)-1-Hydroxycryptotanshinone anhydride
Catalog No.:BCX0305
CAS No.:2301967-86-0
- Spicatanol
Catalog No.:BCX0304
CAS No.:1158208-05-9
- Tanshinol B
Catalog No.:BCX0303
CAS No.:189290-30-0
- Yunnancoronarin C
Catalog No.:BCX0302
CAS No.:260406-45-9
- Hexadecyl (E)-ferulate
Catalog No.:BCX0315
CAS No.:158306-36-6
- Calycosin 7-O-xylosylglucoside
Catalog No.:BCX0316
CAS No.:224957-12-4
- Peruvianoside II
Catalog No.:BCX0317
CAS No.:450407-31-5
- ent-19-Hydroxy-16α,17-isopropylidenedioxyatisan-3-one
Catalog No.:BCX0318
CAS No.:2056274-45-2
- ent-11β,18-Dihydroxy-16α,17-isopropylidenedioxyatisan-3-one
Catalog No.:BCX0319
CAS No.:2056274-46-3
- Nardochalaristolone B
Catalog No.:BCX0320
CAS No.:2251716-60-4
- Secoaristolenedioic acid
Catalog No.:BCX0321
CAS No.:2756011-83-1
- Peruvianoside I
Catalog No.:BCX0322
CAS No.:450407-24-6
- Macrophylloside A
Catalog No.:BCX0323
CAS No.:179457-66-0
- Trijugin D
Catalog No.:BCX0324
CAS No.:1233534-47-8
- Macrophylloside B
Catalog No.:BCX0325
CAS No.:179457-67-1
- Trijugin F
Catalog No.:BCX0326
CAS No.:1233534-51-4
Stilbenes with potent cytotoxicity from the seedcases of Paeonia suffruticosa Andrews.[Pubmed:36403670]
Phytochemistry. 2023 Jan;205:113515.
Stilbenes (based on the 1,2-diphenylethylene skeleton) are a class of plant polyphenols with rich structural and bioactive diversity. Twenty-six stilbenes, including five undescribed compounds (7,8-dioxy-4,3',5'-trihydroxystilbene, trans-13'-methoxygnetin H, suffruticosol E, paestibenetrimerols A and B), were isolated from the seedcases of Paeonia suffruticosa Andrews. Their structures were elucidated by spectroscopic analyses and comparison with previously reported data. The absolute configurations of trans-13'-methoxygnetin H, suffruticosol E, paestibenetrimerols A and B were assigned from their respective electronic circular dichroism (ECD) spectra. Additionally, the structures of known compounds suffruticosols A, B and rockiol B were revised and the absolute configurations of them, and along with (+)-Davidiol A, were also further determined by ECD. The isolated compounds, trans-gnetin H, cis-gnetin H and suffruticosol E, were found to have potent cytotoxicity against the DU-145 and MDA-MB-231 cell lines with IC(50) values of 4.89-8.61 muM. The preliminary antitumor structure-activity relationship of these stilbenes is discussed as well.
Polyphenols isolated from leaves of Vitis thunbergii var. taiwaniana regulate APP related pathway.[Pubmed:26675439]
Bioorg Med Chem Lett. 2016 Jan 15;26(2):505-511.
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by the accumulation of amyloid plaques and neurofibrillary tangles in the brain. The major component of the plaques, amyloid-beta (Abeta), is generated from amyloid-beta precursor protein (APP) by beta- and gamma-secretase-mediated cleavages. Multiple lines of evidence demonstrate that overproduction/accumulation of Abeta in vulnerable brain regions is a primary cause of the pathogenesis of AD. Among the twelve polyphenols isolated from the leaf extracts of Vitis thunbergii var. taiwaniana (VTT), stenophyllol C, stenophyllol B, ampelopsin C, vitisin A, and Davidiol A were shown to significantly reduce both Abeta40 and Abeta42 levels in N2a695 cells. Further studies revealed that ampelopsin C and vitisin A reduce Abeta production through inhibiting beta-secretase activity, while the effects of the other active polyphenols on reducing Abeta generation are through different mechanisms. These results suggest that VTT extracts may be beneficial for AD prevention and treatment.
Viniphenol A, a complex resveratrol hexamer from Vitis vinifera stalks: structural elucidation and protective effects against amyloid-beta-induced toxicity in PC12 cells.[Pubmed:24521157]
J Nat Prod. 2014 Feb 28;77(2):213-7.
Stilbenes have received much attention during the last two decades following the discovery of resveratrol in wine. Since then, there have been a growing number of papers reporting various biological activities of naturally occurring stilbenes. The aim of this study was to determine new minor stilbenes from Vitis vinifera stalks. Purification of these compounds was achieved by means of centrifugal partition chromatography, a versatile separation technique that does not require a solid stationary phase. Viniphenol A (1), a new resveratrol hexamer, was isolated along with five oligostilbenoids identified in V. vinifera for the first time, ampelopsin C, Davidiol A, leachianol F, leachianol G, and E-maackin, a dimer with an unusual dioxane moiety, and 14 known hydroxystilbenes. The structure and stereochemistry of viniphenol A were determined on the basis of spectroscopic data analysis and molecular modeling under NMR constraints. Viniphenol A showed protective effects against amyloid-beta-induced toxicity in PC12 cell cultures.


