DemethoxymatteucinolCAS# 56297-79-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56297-79-1 | SDF | Download SDF |
| PubChem ID | 180550 | Appearance | Yellow powder |
| Formula | C17H16O4 | M.Wt | 284.31 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
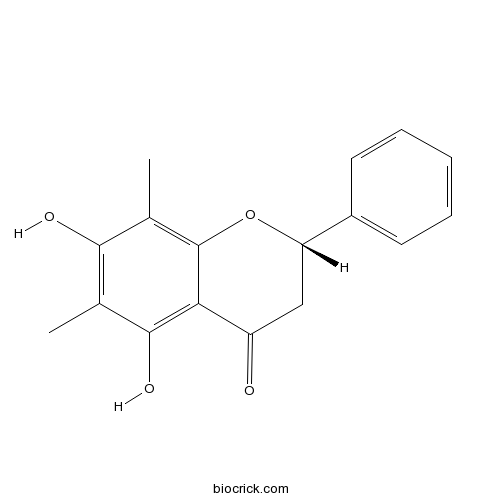
| Chemical Name | (2S)-5,7-dihydroxy-6,8-dimethyl-2-phenyl-2,3-dihydrochromen-4-one | ||
| SMILES | CC1=C(C(=C2C(=C1O)C(=O)CC(O2)C3=CC=CC=C3)C)O | ||
| Standard InChIKey | HAIHGFWQOPJMPV-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C17H16O4/c1-9-15(19)10(2)17-14(16(9)20)12(18)8-13(21-17)11-6-4-3-5-7-11/h3-7,13,19-20H,8H2,1-2H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Demethoxymatteucinol Dilution Calculator

Demethoxymatteucinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5173 mL | 17.5864 mL | 35.1729 mL | 70.3457 mL | 87.9322 mL |
| 5 mM | 0.7035 mL | 3.5173 mL | 7.0346 mL | 14.0691 mL | 17.5864 mL |
| 10 mM | 0.3517 mL | 1.7586 mL | 3.5173 mL | 7.0346 mL | 8.7932 mL |
| 50 mM | 0.0703 mL | 0.3517 mL | 0.7035 mL | 1.4069 mL | 1.7586 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3517 mL | 0.7035 mL | 0.8793 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tetrahydroxymethoxychalcone
Catalog No.:BCN9496
CAS No.:197227-39-7
- Callosin
Catalog No.:BCN9495
CAS No.:166197-43-9
- Phrymarolin V
Catalog No.:BCN9494
CAS No.:1449376-84-4
- 5-Hydroxy-3,6,7,4'-tetramethoxyflavone
Catalog No.:BCN9493
CAS No.:14787-34-9
- Chrysosplenol C
Catalog No.:BCN9492
CAS No.:23370-16-3
- 2,4,3',4',6'-Penta-O-(3-methylbutanoyl)sucrose
Catalog No.:BCN9491
CAS No.:150302-84-4
- (-)-Sesamin 2,2'-diol
Catalog No.:BCN9490
CAS No.:1152441-87-6
- Vesticarpan
Catalog No.:BCN9489
CAS No.:69853-46-9
- 24,24-Dimethyl-5α-lanosta-9(11),25-dien-3β-ol
Catalog No.:BCN9488
CAS No.:33762-29-7
- 3-O-Debenzoylzeylenone
Catalog No.:BCN9487
CAS No.:1800008-77-8
- Methoxyimbricatin
Catalog No.:BCN9486
CAS No.:233759-30-3
- Flaccidin
Catalog No.:BCN9485
CAS No.:115531-76-5
- Glycocitrine I
Catalog No.:BCN9498
CAS No.:82354-36-7
- N-Methylatalaphylline
Catalog No.:BCN9499
CAS No.:28233-34-3
- Swietenidine B
Catalog No.:BCN9500
CAS No.:2721-56-4
- Trigothysoid P
Catalog No.:BCN9501
CAS No.:1501943-10-7
- Koenimbine
Catalog No.:BCN9502
CAS No.:21087-98-9
- 3,4,3'-Tri-O-methylflavellagic acid
Catalog No.:BCN9503
CAS No.:13756-49-5
- 3,2'-Dihydroxy-4,5-dimethoxybibenzyl
Catalog No.:BCN9504
CAS No.:212116-72-8
- Isomurrayafoline B
Catalog No.:BCN9505
CAS No.:107903-15-1
- Diosbulbin E
Catalog No.:BCN9506
CAS No.:67567-14-0
- Pleionesin C
Catalog No.:BCN9507
CAS No.:1222077-25-9
- Deacetylpleionesin C
Catalog No.:BCN9508
CAS No.:1454585-39-7
- Isoengeletin
Catalog No.:BCN9509
CAS No.:30987-58-7
Isolation of 5,7-Dihydroxy, 6,8-Dimethyl Flavanone from Syzygium aqueum with Its Antioxidant and Xanthine Oxidase Inhibitor Activities.[Pubmed:29568189]
Pharmacognosy Res. 2018 Jan-Mar;10(1):60-63.
Background: Syzygium aqueum Burm.f. Alston (water apple) belonging to Myrtaceae family was originated from tropical areas. It was traditionally used as a medicinal plant. Objective: The objective of the study was to isolate the active compound from the methanolic extract of S. aqueum leaves. Methods: Extraction was done using continuous extraction with methanol as a solvent. The extract was then fractionated using liquid-liquid extraction, vacuum liquid chromatography, and radial chromatography. Recrystallization was done for purification. The structure of the compound was determined by Ultraviolet-Visible and (1D and 2D) nuclear magnetic resonance (NMR) spectrometer. Results: The isolate showed maximum wavelengths at 347 (band I) and 296 (band II) nm. After addition of NaOH and CH3 COONa, the maximum wavelengths of band II moved to 340 and 339 nm, respectively. There was no change in wavelengths after addition CH3 COONa/H3 BO3 and AlCl3. The (1)H-NMR spectrum showed 16 protons, whereas (13)C-NMR spectrum showed 15 carbons. Based on those data, the isolate was determined as 5,7-dihydroxy-6,8-dimethyl flavanone (Demethoxymatteucinol). At a concentration of 100 and 50 mug/mL, it could inhibit 25.13% of xanthine oxidase (XO) activity and scavenge 11.87% of diphenyl-picrylhydrazyl, respectively. Conclusion: Demethoxymatteucinol was isolated for the first time from S. aqueum and it had mild antioxidant and XO inhibitory activities. SUMMARY: One flavonoid compound, which 5,7-dihydroxy 6,8-dimethyl flavanone (Demethoxymatteucinol), was isolated from the methanol extract of Syzygium aqueum. It had mild antioxidant and xanthine oxidase inhibitory activities. Abbreviations Used: CH3 COONa/H3 BO3: Natrium acetate/Boric acid; DPPH: Diphenyl-picrylhydrazyl, NMR: Nuclear Magnetic Resonance; ABTS: 2,2'-azinobis (3-ethyl-benzothiazline-6-sulfonic acid); AEAC: Ascorbic Acid Equivalent Antioxidant Capacity; UV-Vis: Ultraviolet-Visible; XO: Xanthine Oxidase; HSQC: Heteronuclear Single Quantum Coherence; HMBC: (Heteronuclear Multiple Bond Correlation).
Taxiphyllin 6'-O-gallate, actinidioionoside 6'-O-gallate and myricetrin 2''-O-sulfate from the leaves of Syzygium samarangense and their biological activities.[Pubmed:25273060]
Chem Pharm Bull (Tokyo). 2014;62(10):1013-8.
Three new compounds were isolated from a MeOH extract of the leaves of Syzygium samarangense, one new cyanogenic glucoside, taxiphyllin 6'-O-gallate (1), one new megastigmane glucoside, actinidioionoside 6'-O-gallate (2), and one new sulfated flavonoid rhamnoside, myricetrin 2''-O-sulfate (3), together with 14 known compounds, lupeol (4), Demethoxymatteucinol (5), cryptostrobin (6), betulinic acid (7), beta-sitosterol glucoside (8), 2R-prunasin (9), myrciaphenone A (10), 1-feruloyl-beta-D-glucopyranoside (11), (3S,5R,6R,7E,9S)-3,5,6,9-tetrahydroxymegastigman-7-ene (12), guaijaverin (13), myricetin 4'-methyl ether 3-O-alpha-L-rhamnopyranoside (14), myricetrin (15), gallic acid (16) and actinidioionoside (17). The structures of the new compounds were determined through a combination of spectroscopic, HPLC and chemical analyses.
Two new C-methyl flavanones from the rhizomes and frond bases of Matteuccia struthiopteris.[Pubmed:23944953]
J Asian Nat Prod Res. 2013 Nov;15(11):1163-7.
Two new C-methyl flavanones, (2S)-5,7-dihydroxy-6,8-dimethyl-4'-methoxydihydroflavone-7-O-(6''-O-acetyl)-beta- d-glucopyranoside (1) and (2S)-5,7-dihydroxy-6,8-dimethyldihydroflavone-7-O-(6''-O-acetyl)-beta-d-glucopyra noside (2), together with five known compounds, Demethoxymatteucinol-7-O-beta-d-glucopyranoside (3), matteucinol-7-O-beta-d-glucopyranoside (4), 5,7-dihydroxy-6-methyl-4'-methoxydihydroflavone (5), methoxymatteucin (6), and thunberginol C (7), were first isolated from the EtOH extract of the rhizomes and frond bases of Matteuccia struthiopteris. The structures were established by spectral analyses, mainly HR-ESI-MS and 1D and 2D NMR experiments (COSY, HSQC, and HMBC).
A novel 1-indanone isolated from Uvaria afzelii roots.[Pubmed:19521904]
Nat Prod Res. 2009;23(10):909-15.
Bioactivity-guided fractionations of chloromethylenic extract of the roots of U. afzelii (Annonaceae), using Leishmania donovani and Trypanosoma brucei brucei bioassay, resulted in the isolation of the two known compounds, emorydone (1) and Demethoxymatteucinol (2), previously isolated from the stems, which were characterised from this source. In addition, the novel 1-indanone, afzeliindanone (3), was also isolated. The structure determination of afzeliindanone (3) was elucidated on the basis of spectral data as 4-[4-hydroxy-3-methoxyphenyl]-indan-1-one. This compound is the first 1-indanone derivative isolated from plants.
[Studies on chemical constituents of rhizome of Matteuccia struthiopteris].[Pubmed:18841771]
Zhongguo Zhong Yao Za Zhi. 2008 Jul;33(14):1703-5.
OBJECTIVE: To study the chemical constituents of the rhizome of Matteuccia struthiopteris. METHOD: The constituents were separated and purified by column chromatography with silica gel and Sephadex LH-20. Their structures were identified on the basis of physical and spectral data. RESULT: Six compounds were isolated and identified as Demethoxymatteucinol (1), matteucinol (2), pinosylvin (3), matteuorien (4), pinosylvin 3-O-beta-D-glucopyranoside (5), matteuorienate A (6). CONCLUSION: All Compounds were isolated from this plant for the first time.
A novel antiplasmodial 3',5'-diformylchalcone and other constituents of Friesodielsia obovata.[Pubmed:17691050]
Nat Prod Res. 2007 Sep;21(11):1009-15.
Phytochemical investigation of the stem and root barks of Friesodielsia obovata Benth (Annonaceae) afforded new 3',5'-diformyl-2',4',6'-trihydroxychalcone and N-formyl-7-hydroxyglaucine together with five known compounds, 3',5'-dimethyl-2',4',6'-trihydroxychalcone, (-)-crotepoxide, Demethoxymatteucinol, lawinal, and benzyl benzoate. 3',5'-diformyl-2',4',6'-trihydroxychalcone indicated significant antiplasmodial, cytotoxicity, and larvicidal activities.
Antiprotozoal compounds from Psorothamnus polydenius.[Pubmed:15679330]
J Nat Prod. 2005 Jan;68(1):108-11.
Bioactivity-guided fractionation of the methanolic extract of Psorothamnus polydenius yielded the new chalcone 2,2',4'-trihydroxy-6'-methoxy-3',5'-dimethylchalcone (2), together with six other known compounds, 2',4'-dihydroxy-6'-methoxy-3',5'-dimethylchalcone (1), dalrubone (3), Demethoxymatteucinol (4), eriodictyol (5), and photodalrubone (6a and 6b). This is the first report of chalcones in P. polydenius. The extracts and isolated compounds were tested in vitro for their antiprotozoal activity against Leishmania donovani and Trypanosoma brucei. Chalcones 1 and 2 and dalrubone (3) exhibited leishmanicidal (IC(50) 5.0, 7.5, and 7.5 microg/mL, respectively) and trypanocidal (IC(50) 6.3, 6.8, and 21.6 microg/mL, respectively) properties. Dalrubone (3) displayed 6-fold selectivity for axenic L. donovani parasites over Vero cells. Furthermore, treatment of L. mexicana-preinfected macrophages with chalcones 1 and 2 and dalrubone (3) (12.5, 12.5, and 25 microg/mL, respectively) reduced the number of infected macrophages by at least 96% while posing no toxicity to the host cell.
Antifungal and antibacterial chalcones from Myrica serrata.[Pubmed:8720393]
Planta Med. 1996 Feb;62(1):67-9.
The dichloromethane extract of the leaves of Myrica serrata inhibits growth of Cladosporium cucumerinum, Bacillus subtilis, and Escherichia coli on TLC plates. Activity-guided fractionation led the isolation of 2',4'-dihydroxy-6'-methoxy-3',5'-dimethylchalcone (1), 2',4'-dihydroxy-6'-methoxy-5'-methylchalcone (aurentiacin A) (2), 2',6'-dihydroxy-4'-methoxy-3',5'-dimethyldihydrochalcone (3), 2'-hydroxy-4',6'-dimethoxy-3'-methyldihydrochalcone (4), and 2', 6'-dihydroxy-4'-methoxy-3'-methyldihydrochalcone (5). In addition, the flavanones Demethoxymatteucinol (6) and cryptostrobin (7) were also identified.
2'-Hydroxymatteucinol, a new C-methyl flavanone derivative from Matteccia orientalis; potent hypoglycemic activity in streptozotocin (STZ)-induced diabetic rat.[Pubmed:8281576]
Chem Pharm Bull (Tokyo). 1993 Oct;41(10):1790-5.
The CHCl3 extract of Matteccia orientalis showed very strong hypoglycemic activity in streptozotocin (STZ)-induced diabetic rats. A new C-methyl flavanone derivative, 2'-hydroxymatteucinol (3) was isolated from the hypoglycemic activity bearing fraction, along with two known compounds, Demethoxymatteucinol (1) and matteucinol (2). The structures of these isolated compounds were elucidated by spectroscopic methods. One of the compounds isolated from CHCl3 extract, 2'-hydroxymatteucinol (3), showed dose-dependent hypoglycemic activity, and a blood sugar lowering effect was observed even at the dose of 10 mg/kg (p.o.) in STZ-induced diabetic rats.


