GR 64349Potent, selective NK2 agonist CAS# 137593-52-3 |
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 137593-52-3 | SDF | Download SDF |
| PubChem ID | 3036081 | Appearance | Powder |
| Formula | C42H68N10O11S | M.Wt | 921.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in 20% ethanol / water | ||
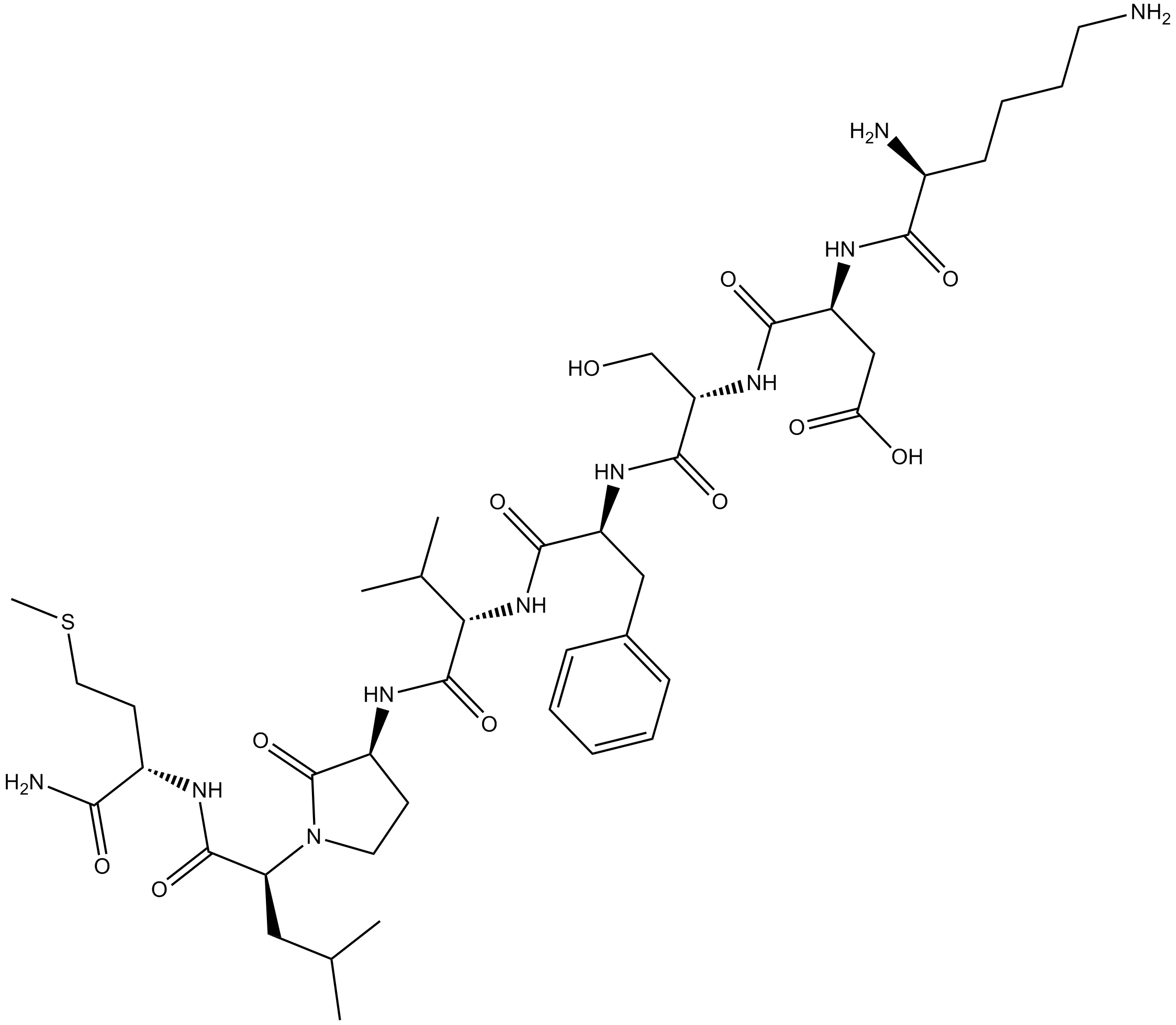
| Sequence | KDSFVGLM (Modifications: Leu-7 = R-γ-lactam-Leu, Met-8 = C-terminal amide) | ||
| Chemical Name | (3S)-4-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(3R)-1-[(2S)-1-[[(2S)-1-amino-4-methylsulfanyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]-2-oxopyrrolidin-3-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-[[(2S)-2,6-diaminohexanoyl]amino]-4-oxobutanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CCSC)C(=O)N)N1CCC(C1=O)NC(=O)C(C(C)C)NC(=O)C(CC2=CC=CC=C2)NC(=O)C(CO)NC(=O)C(CC(=O)O)NC(=O)C(CCCCN)N | ||
| Standard InChIKey | HVUNRXRFMQDMBO-ICQHUDCBSA-N | ||
| Standard InChI | InChI=1S/C42H68N10O11S/c1-23(2)19-32(40(61)46-27(35(45)56)15-18-64-5)52-17-14-28(42(52)63)47-41(62)34(24(3)4)51-38(59)29(20-25-11-7-6-8-12-25)49-39(60)31(22-53)50-37(58)30(21-33(54)55)48-36(57)26(44)13-9-10-16-43/h6-8,11-12,23-24,26-32,34,53H,9-10,13-22,43-44H2,1-5H3,(H2,45,56)(H,46,61)(H,47,62)(H,48,57)(H,49,60)(H,50,58)(H,51,59)(H,54,55)/t26-,27-,28+,29-,30-,31-,32-,34-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective tachykinin NK2 receptor agonist (EC50 = 3.7 nM in rat colon). Displays > 1000- and > 300-fold selectivity over NK1 and NK3 receptors respectively. Active in vivo. |

GR 64349 Dilution Calculator

GR 64349 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bisindolylmaleimide II
Catalog No.:BCC7868
CAS No.:137592-45-1
- Taxifolin 7-O-rhamnoside
Catalog No.:BCN6851
CAS No.:137592-12-2
- [Lys5,MeLeu9,Nle10]-NKA(4-10)
Catalog No.:BCC5994
CAS No.:137565-28-7
- VT-464 racemate
Catalog No.:BCC5399
CAS No.:1375603-36-3
- Poricoic acid B
Catalog No.:BCN8260
CAS No.:137551-39-4
- Poricoic acid A(F)
Catalog No.:BCN3741
CAS No.:137551-38-3
- 1-Benzoylpiperazine
Catalog No.:BCC8456
CAS No.:13754-38-6
- Regiolone
Catalog No.:BCN7193
CAS No.:137494-04-3
- GNE0877
Catalog No.:BCC5369
CAS No.:1374828-69-9
- Rhapontisterone
Catalog No.:BCC8245
CAS No.:137476-71-2
- CO-1686 (AVL-301)
Catalog No.:BCC1490
CAS No.:1374640-70-6
- LEE011 succinate hydrate
Catalog No.:BCC4103
CAS No.:1374639-79-8
- BIM 187
Catalog No.:BCC5933
CAS No.:137734-88-4
- 9-Methoxycanthin-6-one-N-oxide
Catalog No.:BCN2994
CAS No.:137739-74-3
- 7-Methoxy-beta-carboline-1-propionic acid
Catalog No.:BCN2995
CAS No.:137756-13-9
- 12-O-Acetylrosmarinine
Catalog No.:BCN2125
CAS No.:137760-53-3
- Boeravinone E
Catalog No.:BCN4083
CAS No.:137787-00-9
- 2,3-Di(3',4'-methylenedioxybenzyl)-2-buten-4-olide
Catalog No.:BCN1576
CAS No.:137809-97-3
- Valsartan
Catalog No.:BCC5017
CAS No.:137862-53-4
- Valsartan methyl ester
Catalog No.:BCC9189
CAS No.:137863-17-3
- 6-O-Feruloylglucose
Catalog No.:BCN6195
CAS No.:137887-25-3
- ML 239
Catalog No.:BCC3987
CAS No.:1378872-36-6
- Arillatose B
Catalog No.:BCN6196
CAS No.:137941-45-8
- CBB1003
Catalog No.:BCC5524
CAS No.:1379573-88-2
Synthesis of new C-25 and C-26 steroidal acids as potential ligands of the nuclear receptors DAF-12, LXR and GR.[Pubmed:28300583]
Steroids. 2017 May;121:40-46.
A new methodology to obtain C-25 and C-26 steroidal acids starting from pregnenolone is described. Construction of the side chain was achieved by applying the Mukaiyama aldol reaction with a non-hydrolytic work-up to isolate the trapped silyl enol ether with higher yields. Using this methodology we synthesized three new steroidal acids as potential ligands of DAF-12, Liver X and Glucocorticoid nuclear receptors and studied their activity in reporter gene assays. Our results show that replacement of the 21-CH3 by a 20-keto group in the side chains of the cholestane scaffold of DAF-12 or Liver X receptors ligands causes the loss of the activity.
Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexah ydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methano ne (CORT125134): A Selective Glucocorticoid Receptor (GR) Antagonist.[Pubmed:28368581]
J Med Chem. 2017 Apr 27;60(8):3405-3421.
The nonselective glucocorticoid receptor (GR) antagonist mifepristone has been approved in the U.S. for the treatment of selected patients with Cushing's syndrome. While this drug is highly effective, lack of selectivity for GR leads to unwanted side effects in some patients. Optimization of the previously described fused azadecalin series of selective GR antagonists led to the identification of CORT125134, which is currently being evaluated in a phase 2 clinical study in patients with Cushing's syndrome.
Recruitment of bone marrow CD11b(+)Gr-1(+) cells by polymeric nanoparticles for antigen cross-presentation.[Pubmed:28317931]
Sci Rep. 2017 Mar 20;7:44691.
The objective of this study was to investigate the function of poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) on the activation of antigen-specific CD8(+) T cell responses via the CD11b(+)Gr(-)1(+) myeloid subpopulations in murine bone marrow (BM). PLGA NPs containing ovalbumin (OVA) were fabricated by the double-emulsion method. The CD11b(+)Gr-1(low)Ly-6C(high) and CD11b(+)Gr-1(high)Ly-6C(low) subsets from mice bone marrow were sorted and treated with the PLGA/OVA NPs, followed by co-culture with the carboxyfluorescein succinimidyl ester (CFSE)-labelled OT-I CD8(+) cells. Co-culture of OT-I CD8(+) T cells with PLGA/OVA NPs-primed CD11b(+)Gr-1(+) subsets upregulated the expression of IL-2, TNF-alpha, INF-gamma, granzyme B, and perforin, resulting in proliferation of CD8(+) T cells and differentiation into effector cytotoxic T lymphocytes (CTLs). In vivo proliferation of CFSE-labelled OT-I CD8(+) cells in response to OVA was also obtained in the animals immunized with PLGA/OVA NPs. The results presented in this study demonstrate the ability of polymeric NPs to recruit two CD11b(+)Gr(-)1(+) myeloid subsets for effective presentation of exogenous antigen to OT-I CD8(+) T cells in the context of major histocompatibility complex (MHC) class I, leading to an induction of antigen-specific cell proliferation and differentiation into effector cells.
Muscle-specific downregulation of GR levels inhibits adipogenesis in porcine intramuscular adipocyte tissue.[Pubmed:28360421]
Sci Rep. 2017 Mar 30;7(1):510.
Intramuscular adipose is conducive to good pork quality, whereas subcutaneous adipose is considered as waste in pig production. So uncovering the regulation differences between these two adiposes is helpful to tissue-specific control of fat deposition. In this study, we found the sensitivity to glucocorticoids (GCs) was lower in intramuscular adipocytes (IMA) compared with subcutaneous adipocytes (SA). Comparison of glucocorticoid receptor (GR) revealed that IMA had lower GR level which contributed to its reduced GCs sensitivity. Higher methylation levels of GR promotor 1-C and 1-H were detected in IMA compared with SA. GR expression decrease was also found in adipocytes when treated with muscle conditioned medium (MCM) in vitro, which resulted in significant inhibition of adipocytes proliferation and differentiation. Since abundant myostatin (MSTN) was detected in MCM by ELISA assay, we further investigated the effect of this myokine on adipocytes. MSTN treatment suppressed adipocytes GR expression, cell proliferation and differentiation, which mimicked the effects of MCM. The methylation levels of GR promotor 1-C and 1-H were also elevated after MSTN treatment. Our study reveals the role of GR in muscle fiber inhibition on intramuscular adipocytes, and identifies myostatin as a muscle-derived modulator for adipose GR level.
Tachykinin receptor subtypes in the isolated guinea pig heart and their role in mediating responses to neurokinin A.[Pubmed:10871306]
J Pharmacol Exp Ther. 2000 Jul;294(1):147-54.
Selective tachykinin agonists were used to identify cardiac and coronary responses mediated by specific tachykinin receptor subtypes in isolated, perfused guinea pig hearts. Receptor desensitization with selective agonists and blockade with selective antagonists were used to determine the role of specific subtypes in generating responses to neurokinin A (NKA). Dose-dependent cardiac and coronary effects were evoked by bolus injections of inverted question markSar(9), Met(O(2))(11)substance P ( inverted question markSar(9),Met(O(2))(11)SP), GR64349, and inverted question markMePhe(7)neurokinin B ( inverted question markMePhe(7)NKB) (selective agonists for NK(1), NK(2), and NK(3) receptors, respectively). Each agonist caused bradycardia, but GR64349 was most effective (34 +/- 4% decrease in heart rate with 32 nmol, n = 8). Prominent increases in ventricular contractility and perfusion pressure also occurred with 32 nmol of GR64349 (25 +/- 6 and 33 +/- 4%, respectively). inverted question markSar(9), Met(O(2))(11)SP was unique in having a high potency for decreasing ventricular contractility and perfusion pressure. Bolus injections of 25 nmol of NKA decreased rate (48 +/- 2%, n = 51), increased contractility (26 +/- 2%), and had biphasic effects on perfusion pressure (24 +/- 1% decrease followed by 9.2 +/- 1.4% increase). Desensitization with GR64349 or treatment with the NK(2) antagonist SR48968 reduced the bradycardic response to NKA by greater than 75% and eliminated the positive inotropic response. The remaining bradycardia occurred through NK(3) receptors. Desensitization with inverted question markSar(9),Met(O(2))(11)SP or NK(1) blockade with FK888 eliminated the coronary relaxant action of NKA and enhanced the pressor response. It is concluded that three tachykinin receptor subtypes are present in the guinea pig heart and that each contributes to the overall response evoked by NKA.
Endogenous modulation of excitatory amino acid responsiveness by tachykinin NK1 and NK2 receptors in the rat spinal cord.[Pubmed:7582497]
Br J Pharmacol. 1995 Jul;115(6):1013-9.
1. The effects of selective tachykinin (neurokinin, NK) NK1 and NK2 receptor antagonists have been examined on spinal neurones in alpha-chloralose anaesthetized, spinalized rats. They were tested for effects on responses both to excitatory amino acids (EAA) and to noxious heat stimuli. They were also tested for their ability to reverse the actions of selective NK agonists. 2. The NK1-selective antagonists GR82334 (peptide) and CP-99,994 (non-peptide), when applied by microiontophoresis, both reduced responses to kainate > AMPA > NMDA. Intravenous CP-99,994 (3 mg kg-1) also reduced responses to kainate but had inconsistent effects on nociceptive responses. 3. GR82334, applied microiontophoretically, reduced the enhancement by the selective NK1 agonist, GR73632 of both responses to EAAs and background activity. Systemic CP-99,994 (< or = 10 mg kg-1) failed to reverse the effects of GR73632. 4. The selective peptide NK2 antagonist, GR103537, had no consistent effects on responses to EAAs when applied by iontophoresis. In contrast, the non-peptide NK2 antagonist, GR159897, administered systemically (0.5-2 mg kg-1, i.v.) enhanced responses to kainate (but not NMDA); responses to noxious heat were enhanced only weakly. 5. Iontophoretically-administered GR103537 attenuated the effects of the NK2 agonist GR64349, which selectively reduced responses to kainate compared to those to NMDA. Systemically administered GR159897 (< or = 2 mg kg-1, i.v.) caused little antagonism of the effects of GR64349. 6. The data indicate that under these conditions the non-peptide antagonists are not reliable at reversing the actions of selective NK agonists. 7. These results suggest that there is a tonic release of endogenous tachykinins that can modulate glutamatergic neurotransmission in the spinal cord. They provide further support for the hypothesis that release of endogenous NKs acting on NK1 and NK2 receptors can promote NMDA receptor mediated glutamatergic transmission.
Conformationally constrained tachykinin analogues: potent and highly selective neurokinin NK-2 receptor agonists.[Pubmed:1331460]
J Med Chem. 1992 Oct 30;35(22):4195-204.
The design and synthesis of potent and selective neurokinin NK-2 receptor agonists 12 (GR64349) and 31 are described, together with structure-activity relationships for related analogues. Compound 12 (EC50 = 3.7 nM at NK-2 receptors in the rat colon; selectivity > 1000- and > 300-fold with respect to NK-1 and NK-3 receptors, respectively) was derived by incorporation of a Gly-Leu gamma-lactam conformational constraint into the C-terminal region of the neurokinin A octapeptide analogue [Lys3]-NKA(3-10). Compound 31 (EC50 = 15 nM in rat colon) contains a novel fused-bicyclic constraint at the corresponding site in the substance P hexapeptide analogue [Ava6]-SP(6-11).


