RegioloneCAS# 137494-04-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 137494-04-3 | SDF | Download SDF |
| PubChem ID | 44576009 | Appearance | Powder |
| Formula | C10H10O3 | M.Wt | 178.18 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
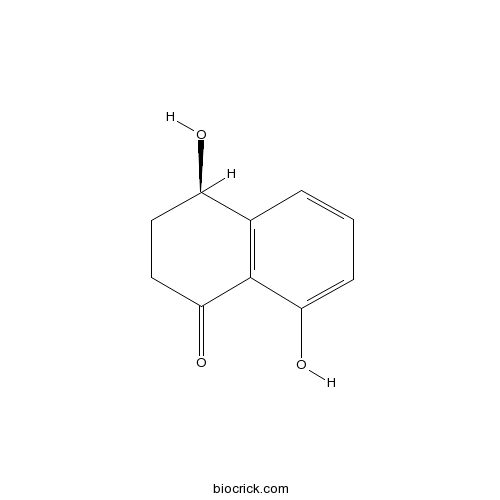
| Chemical Name | (4R)-4,8-dihydroxy-3,4-dihydro-2H-naphthalen-1-one | ||
| SMILES | C1CC(=O)C2=C(C1O)C=CC=C2O | ||
| Standard InChIKey | ZXYYTDCENDYKBR-SSDOTTSWSA-N | ||
| Standard InChI | InChI=1S/C10H10O3/c11-7-4-5-9(13)10-6(7)2-1-3-8(10)12/h1-3,7,11-12H,4-5H2/t7-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Regiolone can induce apoptosis in MCF-7 cells through the caspase-3 independent pathway. 2. Regiolone demonstrates phytotoxicity. |
| Targets | Caspase |

Regiolone Dilution Calculator

Regiolone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6123 mL | 28.0615 mL | 56.123 mL | 112.246 mL | 140.3076 mL |
| 5 mM | 1.1225 mL | 5.6123 mL | 11.2246 mL | 22.4492 mL | 28.0615 mL |
| 10 mM | 0.5612 mL | 2.8062 mL | 5.6123 mL | 11.2246 mL | 14.0308 mL |
| 50 mM | 0.1122 mL | 0.5612 mL | 1.1225 mL | 2.2449 mL | 2.8062 mL |
| 100 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1225 mL | 1.4031 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GNE0877
Catalog No.:BCC5369
CAS No.:1374828-69-9
- Rhapontisterone
Catalog No.:BCC8245
CAS No.:137476-71-2
- CO-1686 (AVL-301)
Catalog No.:BCC1490
CAS No.:1374640-70-6
- LEE011 succinate hydrate
Catalog No.:BCC4103
CAS No.:1374639-79-8
- LEE011 succinate
Catalog No.:BCC4102
CAS No.:1374639-75-4
- BRD4770
Catalog No.:BCC5525
CAS No.:1374601-40-7
- TUG 891
Catalog No.:BCC6235
CAS No.:1374516-07-0
- LY 235959
Catalog No.:BCC6892
CAS No.:137433-06-8
- 15,16-Epoxy-15-ethoxy-6beta,13-dihydroxylabd-8-en-7-one
Catalog No.:BCN7428
CAS No.:1374328-47-8
- Diacerein
Catalog No.:BCN2291
CAS No.:13739-02-1
- Spathulatol
Catalog No.:BCN6877
CAS No.:1373888-27-7
- PF-5274857
Catalog No.:BCC3838
CAS No.:1373615-35-0
- 1-Benzoylpiperazine
Catalog No.:BCC8456
CAS No.:13754-38-6
- Poricoic acid A(F)
Catalog No.:BCN3741
CAS No.:137551-38-3
- Poricoic acid B
Catalog No.:BCN8260
CAS No.:137551-39-4
- VT-464 racemate
Catalog No.:BCC5399
CAS No.:1375603-36-3
- [Lys5,MeLeu9,Nle10]-NKA(4-10)
Catalog No.:BCC5994
CAS No.:137565-28-7
- Taxifolin 7-O-rhamnoside
Catalog No.:BCN6851
CAS No.:137592-12-2
- Bisindolylmaleimide II
Catalog No.:BCC7868
CAS No.:137592-45-1
- GR 64349
Catalog No.:BCC5800
CAS No.:137593-52-3
- BIM 187
Catalog No.:BCC5933
CAS No.:137734-88-4
- 9-Methoxycanthin-6-one-N-oxide
Catalog No.:BCN2994
CAS No.:137739-74-3
- 7-Methoxy-beta-carboline-1-propionic acid
Catalog No.:BCN2995
CAS No.:137756-13-9
- 12-O-Acetylrosmarinine
Catalog No.:BCN2125
CAS No.:137760-53-3
Botrytone, a new naphthalenone pentaketide produced by Botrytis fabae, the causal agent of chocolate spot disease on Vicia faba.[Pubmed:21780794]
J Agric Food Chem. 2011 Sep 14;59(17):9201-6.
A strain of Botrytis fabae isolated from faba bean (Vicia faba L.) plants displaying clear chocolate spot disease symptoms produced phytotoxic metabolites in vitro. The phytotoxins isolated from the culture filtrate organic extract were characterized by spectroscopic and optical methods. A new naphthalenone pentaketide, named botrytone, was isolated and characterized as (4R)-3,4-dihydro-4,5,8-trihydroxy-1(2H)-naphthalenone together with other well-known closely related naphthalenones such as Regiolone and cis- and trans-3,4-dihydro-2,4,8-trihydroxynaphthalen-1(2H)-ones. When tested on leaves of the host plant, with the cis- and trans-3,4-dihydro-2,4,8-trihydroxynaphthalen-1(2H)-ones assayed in mixture, Regiolone demonstrated the highest level of phytotoxicity together with cis- and trans-3,4-dihydro-2,4,8-trihydroxynaphthalen-1(2H)-ones. Botrytone showed moderate phytotoxic activity at 1 mg/mL and was still phytotoxic at 0.5 mg/mL.
Anti-proliferative and apoptotic activities of constituents of chloroform extract of Juglans regia leaves.[Pubmed:24467376]
Cell Prolif. 2014 Apr;47(2):172-9.
OBJECTIVES: To evaluate anti-proliferative as well as apoptotic activities of compounds identified in chloroform extract of Juglans regia leaves, on human breast and oral cancer cell lines (MCF-7 and BHY). MATERIALS AND METHODS: Column chromatography, MTT assay, flowcytometry and western blotting have all been used in the study. RESULTS: Bioassay-guided fractionation of chloroform extract of J. regia afforded isolation of 5-hydroxy-3,7,4'-trimethoxyflavone [1], lupeol [2], daucosterol [3], 4-hydroxy-alpha-tetralone [4], beta-sitosterol [5], 5,7- dihydroxy-3,4'-dimethoxyflavone [6] and Regiolone [7]. Structures of the compounds were established on the basis of spectroscopic analyses [Nuclear magnetic resonance (NMR) and mass]. All compounds inhibited proliferation of MCF-7 (human breast adenocarcinoma) and BHY (human oral squamous carcinoma) cells in a concentration-dependent manner. Compounds 6 and 7 had potent cytotoxic effects on both MCF-7 and BHY cells (IC50 21-51 mum), yet were not toxic to normal cells. MCF-7 growth inhibition was attributed to apoptosis; population of apoptotic cells increased from 1.12% in controls to 5.64 and 8.1% after 48-h treatment with compounds 6 and 7, indicating their potential at inducing early and late apoptosis. The caspase cascade was not activated, as indicated by only insignificant cleavage of caspase-3. CONCLUSIONS: Our results suggest that compounds 6 and 7 can induce apoptosis in MCF-7 cells through the caspase-3 independent pathway.
Aspergchromones A and B, two new polyketides from the marine sponge-associated fungus Aspergillus sp. SCSIO XWS03F03.[Pubmed:28276769]
J Asian Nat Prod Res. 2017 Jul;19(7):684-690.
Two new polyketides, aspergchromones A (1) and B (2), together with five known compounds, secalonic acid D (3), noreugenin (4), (3S)-5-hydroxymellein (5), (4S)-6-hydroxyisosclerone (6), and (-)-Regiolone (7), were isolated from the ethyl acetate extract of marine sponge-derived fungus Aspergillus sp. SCSIO XWS03F03. Their structures were elucidated by means of spectroscopic techniques (1D and 2D NMR, MS, UV, and IR). The absolute configurations of the new compounds were established by ECD calculations. Compound 3 showed moderate antimicrobial activity.
Enantioselective Separation of 4,8-DHT and Phytotoxicity of the Enantiomers on Various Plant Species.[Pubmed:27110760]
Molecules. 2016 Apr 22;21(4):528.
As a candidate for bioherbicide, 4,8-dihydroxy-1-tetralone (4,8-DHT) was isolated from Caryospora callicarpa epicarp and its two enantiomers, S-(+)-isosclerone and R-(-)-Regiolone, were separated by chiral high-performance liquid chromatography (HPLC) on a Chiralcel OD column with chiral stationary phase (CSP)-coated cellulose-tris(3,5-dimethylphenylcarbamate). Then, the phytotoxicity of 4,8-DHT and its enantiomers toward the seeds germination and seedling growth of the five tested plant species, including lettuce (Latuca sativa), radish (Raphanus sativus), cucumber (Cucumis sativus), onion (Allium cepa), and wheat (Triticum aestivum), were investigated and the results indicated a hormesis at low concentration of 4,8-DHT and its enantiomers, but a retardant effect at high concentration. Between the two enantiomers of 4,8-DHT, the S-(+)-isosclerone was more toxic to seeds germination and seedling growth of the five tested plant species than the R-(-)-Regiolone, and also the phytotoxicity of S-(+)-isosclerone varied with different plants. For example, S-(+)-isosclerone was the most active to seedling growth of lettuce, indicating that S-(+)-isosclerone had specific effects on different organisms. Thus, all of the chirality and concentration of 4,8-DHT, as well as the affected plant species, need to be taken into consideration in the development and utilization of 4,8-DHT.


