HyperxanthoneCAS# 99481-41-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99481-41-1 | SDF | Download SDF |
| PubChem ID | 14757909 | Appearance | Powder |
| Formula | C18H14O5 | M.Wt | 310.3 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
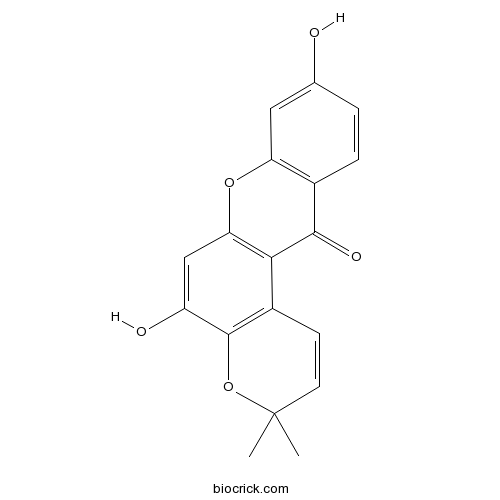
| Chemical Name | 5,9-dihydroxy-3,3-dimethylpyrano[3,2-a]xanthen-12-one | ||
| SMILES | CC1(C=CC2=C(O1)C(=CC3=C2C(=O)C4=C(O3)C=C(C=C4)O)O)C | ||
| Standard InChIKey | GQAYMLKLFVVQQH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H14O5/c1-18(2)6-5-11-15-14(8-12(20)17(11)23-18)22-13-7-9(19)3-4-10(13)16(15)21/h3-8,19-20H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hyperxanthone Dilution Calculator

Hyperxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2227 mL | 16.1134 mL | 32.2269 mL | 64.4538 mL | 80.5672 mL |
| 5 mM | 0.6445 mL | 3.2227 mL | 6.4454 mL | 12.8908 mL | 16.1134 mL |
| 10 mM | 0.3223 mL | 1.6113 mL | 3.2227 mL | 6.4454 mL | 8.0567 mL |
| 50 mM | 0.0645 mL | 0.3223 mL | 0.6445 mL | 1.2891 mL | 1.6113 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3223 mL | 0.6445 mL | 0.8057 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5-Carboxystrictosidine
Catalog No.:BCN9408
CAS No.:34371-47-6
- 2,7-Dihydroxyxanthone
Catalog No.:BCN9407
CAS No.:64632-72-0
- Mumeose K
Catalog No.:BCN9406
CAS No.:2132384-01-9
- Tyramine hydrochloride
Catalog No.:BCN9405
CAS No.:60-19-5
- Symphonone I
Catalog No.:BCN9404
CAS No.:1235774-18-1
- Platachromone A
Catalog No.:BCN9403
CAS No.:1606149-62-5
- Methoxyeugenol
Catalog No.:BCN9402
CAS No.:6627-88-9
- 3β-Isodihydrocadambine
Catalog No.:BCN9401
CAS No.:62014-69-1
- p-Hydroxybenzaldehyde glucoside
Catalog No.:BCN9400
CAS No.:26993-16-8
- Batatasin I
Catalog No.:BCN9399
CAS No.:51415-00-0
- 10-Dehydrogingerdione
Catalog No.:BCN9398
CAS No.:99742-04-8
- Apocynoside I
Catalog No.:BCN9397
CAS No.:358721-31-0
- Physaminimin D
Catalog No.:BCN9410
CAS No.:1582259-05-9
- Physaminimin C
Catalog No.:BCN9411
CAS No.:1582259-03-7
- Methyl (9Z,11E)-13-hydroxyoctadeca-9,11-dienoate
Catalog No.:BCN9412
CAS No.:109837-85-6
- Withaphysalin S
Catalog No.:BCN9413
CAS No.:949172-13-8
- Withaphysalin E
Catalog No.:BCN9414
CAS No.:118985-24-3
- Withaphysalin A
Catalog No.:BCN9415
CAS No.:57423-72-0
- Minisecolide C
Catalog No.:BCN9416
CAS No.:1967030-77-8
- 2,6-Dihydroxyxanthone
Catalog No.:BCN9417
CAS No.:838-11-9
- Dihydromikanolide
Catalog No.:BCN9418
CAS No.:23758-04-5
- 4,5-Dihydropiperlonguminine
Catalog No.:BCN9419
CAS No.:23512-53-0
- Deoxymikanolide
Catalog No.:BCN9420
CAS No.:23753-57-3
- Macluraxanthone
Catalog No.:BCN9421
CAS No.:5848-14-6
Phenolic constituents with neuroprotective activities from Hypericum wightianum.[Pubmed:31229788]
Phytochemistry. 2019 Sep;165:112049.
Five undescribed phenolic compounds, inclusing a depsidone derivative, hyperwightin A, a flavone derivative, hyperwightin B, and three benzophenone glycosides, hyperwightins C-E, along with four known ones were isolated from the 95% EtOH extract of the whole plants of Hypericum wightianum. Structures of the obtained compounds were elucidated by spectroscopic analyses. The protective effects of the isolates against corticosterone-induced PC12cell injury were assessed. Hyperwightin E, petiolin G and Hyperxanthone exhibited noticeable neuroprotection at 10muM.
Molecular Cloning and Characterization of a Xanthone Prenyltransferase from Hypericum calycinum Cell Cultures.[Pubmed:26343621]
Molecules. 2015 Aug 27;20(9):15616-30.
In plants, prenylation of metabolites is widely distributed to generate compounds with efficient defense potential and distinct pharmacological activities profitable to human health. Prenylated compounds are formed by members of the prenyltransferase (PT) superfamily, which catalyze the addition of prenyl moieties to a variety of acceptor molecules. Cell cultures of Hypericum calycinum respond to elicitor treatment with the accumulation of the prenylated xanthone Hyperxanthone E. A cDNA encoding a membrane-bound PT (HcPT) was isolated from a subtracted cDNA library and transcript preparations of H. calycinum. An increase in the HcPT transcript level preceded Hyperxanthone E accumulation in cell cultures of H. calycinum treated with elicitor. The HcPT cDNA was functionally characterized by expression in baculovirus-infected insect cells. The recombinant enzyme catalyzed biosynthesis of 1,3,6,7-tetrahydroxy-8-prenylxanthone through regiospecific C-8 prenylation of 1,3,6,7-tetrahydroxyxanthone, indicating its involvement in Hyperxanthone E formation. The enzymatic product shared significant structural features with the previously reported cholinesterase inhibitor gamma-mangostin. Thus, our findings may offer a chance for semisynthesis of new active agents to be involved in the treatment of Alzheimer's disease.
Cinnamate:CoA ligase initiates the biosynthesis of a benzoate-derived xanthone phytoalexin in Hypericum calycinum cell cultures.[Pubmed:22992510]
Plant Physiol. 2012 Nov;160(3):1267-80.
Although a number of plant natural products are derived from benzoic acid, the biosynthesis of this structurally simple precursor is poorly understood. Hypericum calycinum cell cultures accumulate a benzoic acid-derived xanthone phytoalexin, Hyperxanthone E, in response to elicitor treatment. Using a subtracted complementary DNA (cDNA) library and sequence information about conserved coenzyme A (CoA) ligase motifs, a cDNA encoding cinnamate:CoA ligase (CNL) was isolated. This enzyme channels metabolic flux from the general phenylpropanoid pathway into benzenoid metabolism. HcCNL preferred cinnamic acid as a substrate but failed to activate benzoic acid. Enzyme activity was strictly dependent on the presence of Mg(2)(+) and K(+) at optimum concentrations of 2.5 and 100 mM, respectively. Coordinated increases in the Phe ammonia-lyase and HcCNL transcript levels preceded the accumulation of Hyperxanthone E in cell cultures of H. calycinum after the addition of the elicitor. HcCNL contained a carboxyl-terminal type 1 peroxisomal targeting signal made up by the tripeptide Ser-Arg-Leu, which directed an amino-terminal reporter fusion to the peroxisomes. Masking the targeting signal by carboxyl-terminal reporter fusion led to cytoplasmic localization. A phylogenetic tree consisted of two evolutionarily distinct clusters. One cluster was formed by CoA ligases related to benzenoid metabolism, including HcCNL. The other cluster comprised 4-coumarate:CoA ligases from spermatophytes, ferns, and mosses, indicating divergence of the two clades prior to the divergence of the higher plant lineages.
New phenolic principles from Hypericum sampsonii.[Pubmed:15256712]
Chem Pharm Bull (Tokyo). 2004 Jul;52(7):866-9.
Using the anti-hepatitis B virus (HBV)-producing cell line MS-G2 in vitro cultural system-guided screening was performed, and two new benzophenones, 2,6-dihydroxy-4-[(E)-5-hydroxy-3,7-dimethylocta-2,7-dienyloxy]benzophenone (1) and 2,6-dihydroxy-4-[(E)-7-hydroxy-3,7-dimethylocta-2-enyloxy]benzophenone (2), a new xanthone, Hyperxanthone (3), a new bisanthraquinone glycoside, R-(-)-skyrin-6-O-beta-D-xylopyranoside (4), and 2-caffeoyloxy-3-hydroxy-3-(3,4-dihydroxyphenyl)propyl alcohol (5), and 16 known compounds were isolated from the anti-HBV active fraction of the whole herbs of Hypericum sampsonii. Their structures were elucidated using spectroscopic methods, mainly 2D NMR and MS spectrometry. Circular dichroism was used to determine the stereochemistry of bisanthraquinone glycosides.


