IsoliquiritigeninCAS# 961-29-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 961-29-5 | SDF | Download SDF |
| PubChem ID | 638278 | Appearance | Yellow powder |
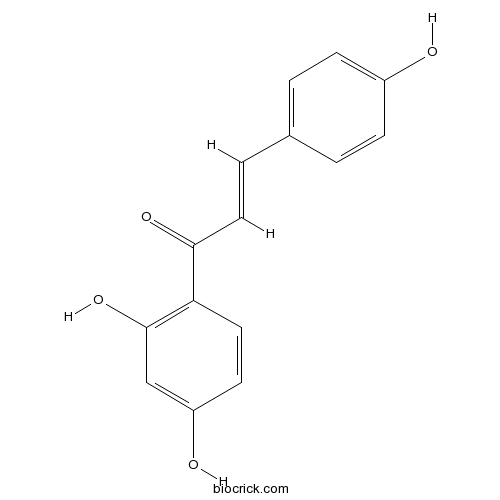
| Formula | C15H12O4 | M.Wt | 256.3 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Synonyms | GU17; ISL; Isoliquiritigen | ||
| Solubility | Ethanol : 100 mg/mL (390.24 mM; Need ultrasonic) DMSO : ≥ 100 mg/mL (390.24 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one | ||
| SMILES | C1=CC(=CC=C1C=CC(=O)C2=C(C=C(C=C2)O)O)O | ||
| Standard InChIKey | DXDRHHKMWQZJHT-FPYGCLRLSA-N | ||
| Standard InChI | InChI=1S/C15H12O4/c16-11-4-1-10(2-5-11)3-8-14(18)13-7-6-12(17)9-15(13)19/h1-9,16-17,19H/b8-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isoliquiritigenin has hepatoprotective, chemopreventive, antitumor, vasorelaxant, anti-platelet, anti-allergic, antiviral, antioxidant and anti-inflammatory effects, it can induce growth inhibition and apoptosis through downregulating AA metabolic network and the deactivation of PI3K/Akt in human breast cancer. Isoliquiritigenin also has the ability to protect cells from AA+iron-induced H2O2 production and mitochondrial dysfunction, which is mediated with GSK3β phosphorylation downstream of AMPK. |
| Targets | Nrf2 | PGE | PI3K | Akt | AMPK | COX | PARP | P450 (e.g. CYP17) | IL Receptor | TNF-α | p65 | NF-kB | IkB | ERK | JNK | GSK-3 | NOS | Sodium Channel | ATPase | Potassium Channel | SOD | IKK |
| In vitro | A protective mechanism of licorice (Glycyrrhiza uralensis): isoliquiritigenin stimulates detoxification system via Nrf2 activation.[Pubmed: 25557030]J Ethnopharmacol. 2015 Mar 13;162:134-9.Licorice (Glycyrrhizae radix), the root of Glycyrrhiza uralensis Fisch. (Leguminosae), is mainly used to moderate the characteristics of toxic herbs in Traditional Chinese Medicine, which could be partly interpreted as detoxification. However, the underlying mechanism is still not fully elucidated. Nuclear factor erythroid 2-related factor 2 (Nrf2) plays a key role in the protection against toxic xenobiotics. In our previous research, we have identified that extracts from Glycyrrhiza uralensis induced the expression of Nrf2 nuclear protein and its downstream genes. This research aims to screen the most potent Nrf2 inducer isolated from Glycyrrhiza uralensis and examine its effect on Nrf2 signaling pathway and detoxification system.
Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages.[Pubmed: 18295200]Eur J Pharmacol. 2008 Apr 14;584(1):175-84.In this study, the anti-inflammatory effects of flavonoids isolated from the roots of Glycyrrhiza uralensis (Leguminosae), namely, isoliquiritin (the glycoside of isoliquirigenin) and Isoliquiritigenin (the aglycone of isoliquiritin) were evaluated on lipopolysaccharide (LPS)-treated RAW 264.7 macrophages. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress.[Pubmed: 20026081 ]Biochem Pharmacol. 2010 May 1;79(9):1352-62.Isoliquiritigenin (ILQ), a flavonoid compound originated from Glycyrrhiza species, is known to activate SIRT1. Arachidonic acid (AA) in combination with iron (a catalyst of auto-oxidation) leads cells to produce excess reactive species with a change in mitochondrial permeability transition. |
| In vivo | In Vivo Gastroprotective Effect along with Pharmacokinetics, Tissue Distribution and Metabolism of Isoliquiritigenin in Mice.[Pubmed: 25875506]Planta Med. 2015 May;81(7):586-93.As numerous herbal products have been used as dietary supplements or functional foods, the demands of the pharmacokinetic and pharmacodynamic characteristics of active compounds are increasing in order to secure a consistent outcome (i.e., efficiency and safety). |
| Kinase Assay | Isoliquiritigenin showed strong inhibitory effects towards multiple UDP-glucuronosyltransferase (UGT) isoform-catalyzed 4-methylumbelliferone (4-MU) glucuronidation.[Pubmed: 23237733]Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of PI3K/Akt in human breast cancer.[Pubmed: 23747687]Toxicol Appl Pharmacol. 2013 Oct 1;272(1):37-48.Arachidonic acid (AA)-derived eicosanoids and its downstream pathways have been demonstrated to play crucial roles in growth control of breast cancer. Fitoterapia. 2013 Jan;84:208-12.Isoliquiritigenin, a herbal ingredient with chalcone structure, has been speculated to be able to inhibit one of the most drug-metabolizing enzymes (DMEs) UDP-glucuronosyltransferase (UGT). |
| Animal Research | Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats.[Pubmed: 16459097 ]Isoliquiritigenin attenuates oxidative hepatic damage induced by carbon tetrachloride with or without buthionine sulfoximine.[Pubmed: 25450236]Chem Biol Interact. 2015 Jan 5;225:13-20.Glycyrrhizae radix (G. radix) has been demonstrated to have hepatoprotective properties. This study determined the therapeutic effects of Isoliquiritigenin (isoLQ) in G. radix, against liver injury induced by CCl4 in rats. Pharmacol Res. 2006 Mar;53(3):303-9.Epidemiological studies indicate that the intake of flavonoids is inversely associated with risk of stroke, cardiovascular diseases and cancer. Isoliquiritigenin (ISL), a flavonoid constituent in the root of Glycyrrhiza glabra, is known to have vasorelaxant effect, antioxidant, anti-platelet, anti-tumor, anti-allergic, antiviral activities and estrogenic properties. However, there is no report on the effects of ISL in cerebral ischemia. Evidence demonstrate that the impaired energy metabolism and the excessive generation of reactive oxygen radicals (ROS) contribute to the brain injury associated with cerebral ischemia. |

Isoliquiritigenin Dilution Calculator

Isoliquiritigenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9017 mL | 19.5084 mL | 39.0168 mL | 78.0336 mL | 97.5419 mL |
| 5 mM | 0.7803 mL | 3.9017 mL | 7.8034 mL | 15.6067 mL | 19.5084 mL |
| 10 mM | 0.3902 mL | 1.9508 mL | 3.9017 mL | 7.8034 mL | 9.7542 mL |
| 50 mM | 0.078 mL | 0.3902 mL | 0.7803 mL | 1.5607 mL | 1.9508 mL |
| 100 mM | 0.039 mL | 0.1951 mL | 0.3902 mL | 0.7803 mL | 0.9754 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Isoliquiritigenin is an anti-tumor flavonoid from the root of Glycyrrhiza glabra, which inhibits aldose reductase with an IC50 of 320 nM.
In Vitro:Isoliquiritigenin may prevent diabetic complications through inhibiting rat lens aldose reductase with an IC50 of 320 nM and sorbitol accumulation in human red blood cells with an IC50 of 2.0 μM[1]. Isoliquiritigenin (100 μM) markedly inhibits the hypoxia-induced prolonged TPS and TR90 of cardiomyocytes. Isoliquiritigenin significantly triggers AMPK Thr172 phosphorylation as compared with vehicle group. Isoliquiritigenin treatment also induces extracellular signal-regulated kinase (ERK) signaling pathway in the cardiomyocytes. Isoliquiritigenin treatment significantly decreases the intracellular ROS levels of isolated cardiomyocytes during hypoxia/reoxygenation[3]. Isoliquiritigenin not only downregulates IL-6 expression but also significantly decreases levels of phosphorylated ERK and STAT3 and can inhibit phosphorylation levels of ERK and STAT3 induced by recombinant human IL-6, which are critical signaling proteins in IL-6 signaling regulation networks[4].
In Vivo:Isoliquiritigenin shows concentration-dependent inhibition of the tonic contraction of mouse jejunum induced by various types of stimulants such as CCh (1 mM), KCl (60 mM) and BaCl2 (0.3 mM) with IC50 values of 4.96±1.97 mM, 4.03±1.34 mM and 3.70±0.58 mM, respectively[2]. Isoliquiritigenin exhibits significant anti-tumor activity in MM xenograft models and synergistically enhances the anti-myeloma activity of adriamycin[4].
References:
[1]. Aida K, et al. Isoliquiritigenin: a new aldose reductase inhibitor from glycyrrhizae radix. Planta Med. 1990 Jun;56(3):254-8.
[2]. Sato Y, et al. Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biol Pharm Bull. 2007 Jan;30(1):145-9.
[3]. Zhang X. Natural antioxidant-isoliquiritigenin ameliorates contractile dysfunction of hypoxic cardiomyocytes via AMPK signaling pathway. Mediators Inflamm. 2013;2013:390890.
[4]. Chen X, et al. Isoliquiritigenin inhibits the growth of multiple myeloma via blocking IL-6 signaling. J Mol Med (Berl). 2012 Nov;90(11):1311-9.
- 2'-Deoxyguanosine
Catalog No.:BCC5433
CAS No.:961-07-9
- Stylopine hydrochloride
Catalog No.:BCN6964
CAS No.:96087-21-7
- Massoniresinol
Catalog No.:BCN4511
CAS No.:96087-10-4
- ent-17-Hydroxykauran-3-one
Catalog No.:BCN4510
CAS No.:960589-81-5
- Jatrorrhizine Hydrochloride
Catalog No.:BCC8193
CAS No.:960383-96-4
- ONX-0914 (PR-957)
Catalog No.:BCC2095
CAS No.:960374-59-8
- Meropenem
Catalog No.:BCC2489
CAS No.:96036-03-2
- Vortioxetine (Lu AA21004) HBr
Catalog No.:BCC1213
CAS No.:960203-27-4
- SD 1008
Catalog No.:BCC2442
CAS No.:960201-81-4
- 2-hexyl-4-Pentynoic Acid
Catalog No.:BCC6480
CAS No.:96017-59-3
- Mepivacaine
Catalog No.:BCC9020
CAS No.:96-88-8
- Aminothiazole
Catalog No.:BCC4623
CAS No.:96-50-4
- MPEP
Catalog No.:BCC4594
CAS No.:96206-92-7
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
- Methyl 8-hydroxy-3-(2-methoxy-2-oxoethyl)-6-methyl-9-oxo-9H-furo[3,4-b]chromene-1-carboxylate
Catalog No.:BCN7465
CAS No.:96287-41-1
- Androst-2-en-17-one
Catalog No.:BCC8821
CAS No.:963-75-7
- Huzhangoside D
Catalog No.:BCN2527
CAS No.:96315-53-6
- Metaphit
Catalog No.:BCC5664
CAS No.:96316-00-6
- Neonuezhenide
Catalog No.:BCN7461
CAS No.:96382-91-1
- H-ß-HoLeu-OH.HCl
Catalog No.:BCC3238
CAS No.:96386-92-4
- Fmoc-1-Nal-OH
Catalog No.:BCC3285
CAS No.:96402-49-2
- Goniotriol
Catalog No.:BCN4745
CAS No.:96405-62-8
- Amygdaloside
Catalog No.:BCC8231
CAS No.:96420-61-0
- Goniodiol
Catalog No.:BCN3958
CAS No.:96422-52-5
Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages.[Pubmed:18295200]
Eur J Pharmacol. 2008 Apr 14;584(1):175-84.
In this study, the anti-inflammatory effects of flavonoids isolated from the roots of Glycyrrhiza uralensis (Leguminosae), namely, isoliquiritin (the glycoside of isoliquirigenin) and Isoliquiritigenin (the aglycone of isoliquiritin) were evaluated on lipopolysaccharide (LPS)-treated RAW 264.7 macrophages. Isoliquiritigenin (ILG) more potently inhibited LPS-induced nitric oxide (NO) and prostaglandin E(2) (PGE(2)) production than isoliquiritin (ILT). Consistent with these findings, ILG reduced the LPS-induced expressions of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) at the protein and mRNA levels in a concentration-dependent manner, as determined by Western blotting and RT-PCR, respectively. In addition, the release of tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6), and the mRNA expression levels of these cytokines were reduced by ILG in a dose-dependent manner. Moreover, ILG attenuated the LPS-induced DNA binding activity and the transcription activity of nuclear factor-kappa B (NF-kappaB), and this was associated with a decrease in inhibitory kappa B-alpha (IkappaB-alpha) phosphorylation and in the subsequent blocking of p65 and p50 protein translocations to the nucleus. Furthermore, ILG suppressed the phosphorylations of IkappaB kinase (IKK), ERK1/2, and p38, whereas the phosphorylation of JNK1/2 was unaffected. These results suggest that the anti-inflammatory properties of ILG are caused by iNOS, COX-2, TNF-alpha, and IL-6 down-regulation due to NF-kappaB inhibition via the suppression of IKK, ERK1/2 and p38 phosphorylation in RAW 264.7 cells.
Isoliquiritigenin showed strong inhibitory effects towards multiple UDP-glucuronosyltransferase (UGT) isoform-catalyzed 4-methylumbelliferone (4-MU) glucuronidation.[Pubmed:23237733]
Fitoterapia. 2013 Jan;84:208-12.
Isoliquiritigenin, a herbal ingredient with chalcone structure, has been speculated to be able to inhibit one of the most drug-metabolizing enzymes (DMEs) UDP-glucuronosyltransferase (UGT). Therefore, the aim of the present study was to investigate the inhibition of Isoliquiritigenin towards important UGT isoforms in the liver and intestine, including UGT1A1, 1A3, 1A6, 1A7, 1A8, 1A9 and 1A10. The recombinant UGT-catalyzed 4-methylumbelliferone (4-MU) glucuronidation was used as probe reactions. The results showed that 100muM of Isoliquiritigenin inhibited the activity of UGT1A1, UGT1A3, UGT1A6, UGT1A7, UGT1A8, UGT1A9, and UGT1A10 by 95.2%, 76.1%, 78.9%, 87.2%, 67.2%, 94.8%, and 91.7%, respectively. The data fitting using Dixon plot and Lineweaver-Burk plot showed that the inhibition of UGT1A1, UGT1A9 and UGT1A10 by Isoliquiritigenin was all best fit to the competitive inhibition, and the second plot using the slopes from the Lineweaver-Burk plot versus Isoliquiritigenin concentrations was used to calculate the inhibition kinetic parameter (K(i)) to be 0.7muM, 0.3muM, and 18.3muM for UGT1A1, UGT1A9, and UGT1A10, respectively. All these results indicated the risk of clinical application of Isoliquiritigenin on the drug-drug interaction and other possible diseases induced by the inhibition of Isoliquiritigenin towards these UGT isoforms.
In vivo gastroprotective effect along with pharmacokinetics, tissue distribution and metabolism of isoliquiritigenin in mice.[Pubmed:25875506]
Planta Med. 2015 May;81(7):586-93.
As numerous herbal products have been used as dietary supplements or functional foods, the demands of the pharmacokinetic and pharmacodynamic characteristics of active compounds are increasing in order to secure a consistent outcome (i.e., efficiency and safety). In this study, the pharmacokinetics including tissue distribution, metabolism, and protein binding of Isoliquiritigenin, a chalcone found in Glycyrrhiza glabra, and its metabolite, liquiritigenin, at various doses in mice are reported. Also, correlations between the preferential tissue distribution and pharmacological effect of Isoliquiritigenin in certain organs were investigated using the in vivo gastroprotective effect of Isoliquiritigenin in mice with indomethacin-induced ulcer. The absorbed fraction of Isoliquiritigenin was high, but the absolute bioavailability was low mainly due to its metabolism. In spite of the low bioavailability, the gastroprotective effect of Isoliquiritigenin was attributed to its high distribution in the stomach. Isoliquiritigenin prevented the occurrence of gastric ulcers by indomethacin, which is associated with increased gastric mucous secretion because the pretreatment with Isoliquiritigenin presumably counteracted the decreased cyclooxygenase 2 by indomethacin. This may suggest that the pharmacokinetic properties of Isoliquiritigenin are useful to predict its efficacy as a gastroprotective agent in a target organ such as the stomach.
Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats.[Pubmed:16459097]
Pharmacol Res. 2006 Mar;53(3):303-9.
Epidemiological studies indicate that the intake of flavonoids is inversely associated with risk of stroke, cardiovascular diseases and cancer. Isoliquiritigenin (ISL), a flavonoid constituent in the root of Glycyrrhiza glabra, is known to have vasorelaxant effect, antioxidant, anti-platelet, anti-tumor, anti-allergic, antiviral activities and estrogenic properties. However, there is no report on the effects of ISL in cerebral ischemia. Evidence demonstrate that the impaired energy metabolism and the excessive generation of reactive oxygen radicals (ROS) contribute to the brain injury associated with cerebral ischemia. In the present study, the protective effects of ISL were investigated in transient middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia-reperfusion injury in rats. Male Sprague-Dawley rats were divided into five groups: sham-operated group, vehicle-pretreated group, and three ISL-pretreated groups (5, 10 and 20 mg kg(-1), i.g.). ISL were administered once a day, for 7 days prior to ischemia. The rats were subjected to 2 h right MCAO via the intraluminal filament technique and 22 h reperfusion. Pretreatment with ISL significantly reduced the cerebral infarct volume and edema and produced significant reduction in neurological deficits. In this study, in order to clarify the mechanism of ISL's protection against cerebral ischemia damage, cerebral energy metabolism, brain Na+K+ATPase activity, malondialdehyde (MDA) content and antioxidant enzyme activities were measured. ISL pretreatment increased the brain ATP content, energy charge (EC) and total adenine nucleotides (TAN) in a dose-dependent manner. The brain Na+K+ATPase activity was protected significantly by pretreatment of ISL for 7 days. Pretreatment with ISL significantly inhibited the increases of brain MDA content and prevented the activities of brain superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) from declines caused by cerebral ischemia-reperfusion. All these findings indicate that ISL has the protective potential against cerebral ischemia injury and its protective effects may be due to the amelioration of cerebral energy metabolism and its antioxidant property.
Isoliquiritigenin attenuates oxidative hepatic damage induced by carbon tetrachloride with or without buthionine sulfoximine.[Pubmed:25450236]
Chem Biol Interact. 2015 Jan 5;225:13-20.
Glycyrrhizae radix (G. radix) has been demonstrated to have hepatoprotective properties. This study determined the therapeutic effects of Isoliquiritigenin (isoLQ) in G. radix, against liver injury induced by CCl4 in rats. CCl4 (0.5 ml/kg/d, twice) or CCl4 plus buthionine sulfoximine exerted severe liver damage assessed by increased plasma levels of alanine aminotransferase and aspartate aminotransferase, in addition to hepatic degeneration and necrosis. These pathological changes were markedly protected by pretreatment with isoLQ (5, 20 mg/kg/d, p.o.) for 3 consecutive days. In addition, pretreatment with isoLQ inhibited CCl4-induced reduction of cytochrome P450 2E1 protein and mRNA expression as well as activity in the liver. Moreover, isoLQ pretreatment reversed the decrease in hepatic antioxidant capacity induced by CCl4 as well as suppressed expression of tumor necrosis factor-alpha and cyclooxigenase-2 in the liver. These results suggest that isoLQ has a protective effect against CCl4-induced liver damage through induction of antioxidant and anti-inflammatory activities.
AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress.[Pubmed:20026081]
Biochem Pharmacol. 2010 May 1;79(9):1352-62.
Isoliquiritigenin (ILQ), a flavonoid compound originated from Glycyrrhiza species, is known to activate SIRT1. Arachidonic acid (AA) in combination with iron (a catalyst of auto-oxidation) leads cells to produce excess reactive species with a change in mitochondrial permeability transition. In view of the importance of oxidative stress in cell death and inflammation, this study investigated the potential of ILQ to protect cells against the mitochondrial impairment induced by AA+iron and the underlying basis for this cytoprotection. Treatment with ILQ inhibited apoptosis induced by AA+iron, as evidenced by alterations in the levels of the proteins associated with cell viability: ILQ prevented a decrease in Bcl-x(L), and cleavage of poly(ADP-ribose)polymerase and procaspase-3. Moreover, ILQ inhibited the ability of AA+iron to elicit mitochondrial dysfunction. In addition, superoxide generation in mitochondria was attenuated by ILQ treatment. Consistently, ILQ prevented cellular H2O2 production increased by AA+iron, thereby enabling cells to restore GSH content. ILQ treatment enhanced inhibitory phosphorylation of glycogen synthase kinase-3beta (GSK3beta), and prevented a decrease in the GSK3beta phosphorylation elicited by AA+iron, which contributed to protecting cells and mitochondria. GSK3beta phosphorylation by ILQ was preceded by AMP-activated protein kinase (AMPK) activation, which was also responsible for mitochondrial protection, as shown by reversal of its effect in the experiments using a dominant negative mutant of AMPK and compound C. Moreover, the AMPK activation led to GSK3beta phosphorylation. These results demonstrate that ILQ has the ability to protect cells from AA+iron-induced H2O2 production and mitochondrial dysfunction, which is mediated with GSK3beta phosphorylation downstream of AMPK.
A protective mechanism of licorice (Glycyrrhiza uralensis): isoliquiritigenin stimulates detoxification system via Nrf2 activation.[Pubmed:25557030]
J Ethnopharmacol. 2015 Mar 13;162:134-9.
ETHNOPHARMACOLOGICAL RELEVANCE: Licorice (Glycyrrhizae radix), the root of Glycyrrhiza uralensis Fisch. (Leguminosae), is mainly used to moderate the characteristics of toxic herbs in Traditional Chinese Medicine, which could be partly interpreted as detoxification. However, the underlying mechanism is still not fully elucidated. Nuclear factor erythroid 2-related factor 2 (Nrf2) plays a key role in the protection against toxic xenobiotics. In our previous research, we have identified that extracts from Glycyrrhiza uralensis induced the expression of Nrf2 nuclear protein and its downstream genes. This research aims to screen the most potent Nrf2 inducer isolated from Glycyrrhiza uralensis and examine its effect on Nrf2 signaling pathway and detoxification system. MATERIALS AND METHODS: Four compounds derived from Glycyrrhiza uralensis (glycyrrhetinic acid, liquiritigenin, Isoliquiritigenin and liquiritin) were screened by ARE-luciferase reporter. The most potent ARE-luciferase inducer was chosen to further examine its effect on Nrf2 and detoxification genes in HepG2 cells. The role of Nrf2-dependent mechanism was tested by using Nrf2 knockout mice (Nrf2 KO) and Nrf2 wild-type mice (Nrf2 WT). RESULTS: ARE-luciferase reporter assay showed these four compounds were all potent Nrf2 inducers, and Isoliquiritigenin was the most potent inducer. Isoliquiritigenin significantly up-regulated the expression of Nrf2 and its downstream detoxification genes UDP-glucuronosyltransferase 1A1 (UGT1A1), glutamate cysteine ligase (GCL), multidrug resistance protein 2 (MRP2) and bile salt export pump (BSEP) in vitro and in vivo. Additionally, Isoliquiritigenin showed Nrf2-dependent transactivation of UGT1A1, GCLC and MRP2. CONCLUSIONS: Isoliquiritigenin, isolated from Glycyrrhiza uralensis, stimulates detoxification system via Nrf2 activation, which could be a potential protective mechanism of licorice.
Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of PI3K/Akt in human breast cancer.[Pubmed:23747687]
Toxicol Appl Pharmacol. 2013 Oct 1;272(1):37-48.
Arachidonic acid (AA)-derived eicosanoids and its downstream pathways have been demonstrated to play crucial roles in growth control of breast cancer. Here, we demonstrate that Isoliquiritigenin, a flavonoid phytoestrogen from licorice, induces growth inhibition and apoptosis through downregulating multiple key enzymes in AA metabolic network and the deactivation of PI3K/Akt in human breast cancer. Isoliquiritigenin diminished cell viability, 5-bromo-2'-deoxyuridine (BrdU) incorporation, and clonogenic ability in both MCF-7 and MDA-MB-231cells, and induced apoptosis as evidenced by an analysis of cytoplasmic histone-associated DNA fragmentation, flow cytometry and hoechst staining. Furthermore, Isoliquiritigenin inhibited mRNA expression of multiple forms of AA-metabolizing enzymes, including phospholipase A2 (PLA2), cyclooxygenases (COX)-2 and cytochrome P450 (CYP) 4A, and decreased secretion of their products, including prostaglandin E2 (PGE2) and 20-hydroxyeicosatetraenoic acid (20-HETE), without affecting COX-1, 5-lipoxygenase (5-LOX), 5-lipoxygenase activating protein (FLAP), and leukotriene B4 (LTB4). In addition, it downregulated the levels of phospho-PI3K, phospho-PDK (Ser(241)), phospho-Akt (Thr(308)), phospho-Bad (Ser(136)), and Bcl-xL expression, thereby activating caspase cascades and eventually cleaving poly(ADP-ribose) polymerase (PARP). Conversely, the addition of exogenous eicosanoids, including PGE2, LTB4 and a 20-HETE analog (WIT003), and caspase inhibitors, or overexpression of constitutively active Akt reversed Isoliquiritigenin-induced apoptosis. Notably, Isoliquiritigenin induced growth inhibition and apoptosis of MDA-MB-231 human breast cancer xenografts in nude mice, together with decreased intratumoral levels of eicosanoids and phospho-Akt (Thr(308)). Collectively, these data suggest that Isoliquiritigenin induces growth inhibition and apoptosis through downregulating AA metabolic network and the deactivation of PI3K/Akt in human breast cancer.


