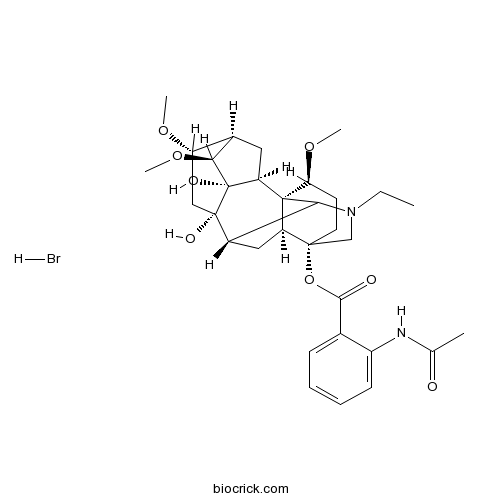
Lappaconitine Hydrobromideanti-inflammatory effects CAS# 97792-45-5 |
- WZ4002
Catalog No.:BCC1074
CAS No.:1213269-23-8
- CO-1686 (AVL-301)
Catalog No.:BCC1490
CAS No.:1374640-70-6
- Mutant EGFR inhibitor
Catalog No.:BCC4119
CAS No.:1421373-62-7
- Erlotinib Hydrochloride
Catalog No.:BCC3645
CAS No.:183319-69-9
- Lapatinib Ditosylate
Catalog No.:BCC2083
CAS No.:388082-78-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 97792-45-5 | SDF | Download SDF |
| PubChem ID | 51346120 | Appearance | Powder |
| Formula | C32H45BrN2O8 | M.Wt | 665.61 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Allapinine;Lappaconite HBr | ||
| Solubility | DMSO : 50 mg/mL (75.12 mM; Need ultrasonic) | ||
| Chemical Name | [(1S,2S,3S,4S,5R,6S,8S,9S,13S,16S,17S)-11-ethyl-3,8-dihydroxy-4,6,16-trimethoxy-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecan-13-yl] 2-acetamidobenzoate;hydrobromide | ||
| SMILES | CCN1CC2(CCC(C34C2CC(C31)C5(CC(C6CC4C5(C6OC)O)OC)O)OC)OC(=O)C7=CC=CC=C7NC(=O)C.Br | ||
| Standard InChIKey | CFFYROOPXPKMEQ-OPLXFBIMSA-N | ||
| Standard InChI | InChI=1S/C32H44N2O8.BrH/c1-6-34-16-29(42-28(36)18-9-7-8-10-21(18)33-17(2)35)12-11-25(40-4)31-23(29)14-20(26(31)34)30(37)15-22(39-3)19-13-24(31)32(30,38)27(19)41-5;/h7-10,19-20,22-27,37-38H,6,11-16H2,1-5H3,(H,33,35);1H/t19-,20+,22+,23-,24+,25+,26?,27+,29-,30+,31+,32+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lappaconitine hydrobromide, a diterpene alkaloid, is a drug for the treatment of cardiac arrhythmias, it is a selective blocker of the TTX-sensitive Na+ channels, and does not influence on the activation threshold of Na+ channels. Lappaconitine hydrobromide can be used for local anesthesia, and analgesic treatment. Lappaconitine hydrobromide has anti-inflammatory effects, it can remove inflammation and swelling, lower temperature and relieve heat. |
| Targets | Immunology & Inflammation related | Sodium Channel |
| In vitro | Pharmacokinetic study of lappaconitine hydrobromide in mice by LC-MS.[Pubmed: 21748973]Yao Xue Xue Bao. 2011 Apr;46(4):432-7.A high sensitive and rapid method was developed for the analysis of lappaconitine in mouse plasma using liquid chromatography coupled to mass spectrometry (LC-MS). |
| Structure Identification | Electrophoresis. 2012 Aug;33(16):2577-83.Simultaneous determination of lappaconitine hydrobromide and isopropiram fumarate in rabbit plasma by capillary electrophoresis with electrochemiluminescence detection.[Pubmed: 22899266]
|

Lappaconitine Hydrobromide Dilution Calculator

Lappaconitine Hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5024 mL | 7.5119 mL | 15.0238 mL | 30.0476 mL | 37.5595 mL |
| 5 mM | 0.3005 mL | 1.5024 mL | 3.0048 mL | 6.0095 mL | 7.5119 mL |
| 10 mM | 0.1502 mL | 0.7512 mL | 1.5024 mL | 3.0048 mL | 3.756 mL |
| 50 mM | 0.03 mL | 0.1502 mL | 0.3005 mL | 0.601 mL | 0.7512 mL |
| 100 mM | 0.015 mL | 0.0751 mL | 0.1502 mL | 0.3005 mL | 0.3756 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lappaconite Hydrobromide (Lappaconite HBr), a kind of alkaloid isolated from Aconitum sinomontanum Nakai and has showed anti-inflammatory effects [1, 2]. Lappaconite HBr acted as an analgesic drug in China. The Aconitum sinomontanum belongs to the Ranunculaceae family and it is used widely as an antirheumatic and analgesic agent in traditional Chinese medicine [1].
In vitro: N/A
In vivo: Lappaconite Hydrobromide absorption percentage in rat stomachs after administration was 9.67%. The absorption percentages at colon, jejunum, ileum and duodenum were, 9.51%, 11.83%, 12.95%, and 19.61%, respectively. The uptake of lappaconite hydrobromide was linearly increased when the concentration was raised from 10 mg/L to 40 mg/L, whereas the absorption rate constant kept at the same level. The LD50 values are of 10.5 mg/kg (i.p. administration) and 9.9 mg/kg (i.p. administration) in mice and rat, respectively. In addition, in anesthetized rabbits injected with lappaconite up to 1 mg/kg, cardiac arrhythmia was quickly observed [3].
References:
1. Xie FM, Wang HC, Li JH, Shu HL, Jiang JR, Chang JP, et al. Studies on the metabolism of lappaconitine in humans. Identification of the metabolites of lappaconitine in human urine by high performance liquid chromatography. Biomed Chromatogr. 1990;4(1):43-6.
2. Guo T, Zhang Y, Zhao J, Zhu C, Feng N. Nanostructured lipid carriers for percutaneous administration of alkaloids isolated from Aconitum sinomontanum. J Nanobiotechnology. 2015;13:47.
3. Shamma M, Chinnasamy P, Miana GA, Khan A, Bashir M, Salazar M, et al. The alkaloids of Delphinium cashmirianum. J Nat Prod. 1979;42(6):615-23.
- Jasmoside
Catalog No.:BCN7552
CAS No.:97763-17-2
- 2,6-Dimethyl-3-O-methyl-4-(2-methylbutyryl)phloroglucinol
Catalog No.:BCN7356
CAS No.:97761-91-6
- 2,6-Dimethyl-3-O-methyl-4-isobutyrylphloroglucinol
Catalog No.:BCN7355
CAS No.:97761-90-5
- S186
Catalog No.:BCC5285
CAS No.:97759-16-5
- Chuanxiongzine hydrochloride
Catalog No.:BCC8147
CAS No.:97747-88-1
- Decynium 22
Catalog No.:BCC6271
CAS No.:977-96-8
- Boldenone propionate
Catalog No.:BCC8895
CAS No.:977-32-2
- Irinotecan
Catalog No.:BCC2490
CAS No.:97682-44-5
- Latrepirdine
Catalog No.:BCC4541
CAS No.:97657-92-6
- Ganoderic acid D2
Catalog No.:BCC8989
CAS No.:97653-94-6
- Lucidone B
Catalog No.:BCN8242
CAS No.:97653-93-5
- Eticlopride hydrochloride
Catalog No.:BCC7193
CAS No.:97612-24-3
- IMD 0354
Catalog No.:BCC4556
CAS No.:978-62-1
- Penciclovir Sodium
Catalog No.:BCC5635
CAS No.:97845-62-0
- 8,9-Didehydro-7-hydroxydolichodial
Catalog No.:BCN6674
CAS No.:97856-19-4
- Norfloxacin lactate
Catalog No.:BCC9104
CAS No.:97867-34-0
- Estradiol valerate
Catalog No.:BCC4482
CAS No.:979-32-8
- 3,4'-Dihydroxy-3,5',7-trimethoxyflavan
Catalog No.:BCN4528
CAS No.:97914-19-7
- Methyl 3-carbazolecarboxylate
Catalog No.:BCN4529
CAS No.:97931-41-4
- Sophoraflavanone G
Catalog No.:BCN2987
CAS No.:97938-30-2
- Leachianone A
Catalog No.:BCN4530
CAS No.:97938-31-3
- Methyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN1294
CAS No.:97966-29-5
- Benzenesulfonic acid
Catalog No.:BCC8846
CAS No.:98-11-3
- 4-Aminophenylarsonic acid
Catalog No.:BCC8688
CAS No.:98-50-0
Pharmacokinetic study of lappaconitine hydrobromide in mice by LC-MS.[Pubmed:21748973]
Yao Xue Xue Bao. 2011 Apr;46(4):432-7.
A high sensitive and rapid method was developed for the analysis of lappaconitine in mouse plasma using liquid chromatography coupled to mass spectrometry (LC-MS). Detection was performed by positive ion electrospray ionization (ESI) in multiple reaction monitoring (MRM) mode, monitoring the transitions m/z 585 --> m/z 535 and m/z 356 --> m/z 192, for the quantification of lappaconitine and tetrahydropalmatine (internal standard, IS), respectively. The method was linear over the concentration range of 3.0-2000.0 ng x mL(-1). The lower limit of quantification was 3.0 ng x mL(-1). Intra- and inter-run precisions (RSD) were both less than 9.9% and accuracy (RE) within +/- 4.8%. After single intravenous injections of Lappaconitine Hydrobromide at 1.0, 2.0 and 4.0 mg x kg(-1), the elimination half-lives (t(1/2)) were 0.47, 0.48 and 0.49 h, and the areas under the curve (AUC(0-t)) were 55.5, 110.5 and 402.9 ng x h x mL(-1), separately. The pharmacokinetic profile of lappaconitine was linear at relatively lower dose levels (1.0-2.0 mg x kg(-1)). When the dose increased farther to 4.0 mg x kg(-1), the Vz and CL decreased, and the increase fold of the AUC was much larger than that of the dose.
Simultaneous determination of lappaconitine hydrobromide and isopropiram fumarate in rabbit plasma by capillary electrophoresis with electrochemiluminescence detection.[Pubmed:22899266]
Electrophoresis. 2012 Aug;33(16):2577-83.
A CE electrochemiluminescence (CE-ECL) method for simultaneous determination of Lappaconitine Hydrobromide (LH) and isopropiram fumarate (IF) has been first established, with a chemically modified platinum electrode by europium (III)-doped Prussian blue analogue film as a working electrode. The conditions for CE separation and ECL detection are discussed and optimized in detail. It has been proved that 20 mmol/L phosphate buffer (pH 8.5) containing 5% (v/v) ACN and 0.17 mol/L SDS could achieve the most favorable resolution, and the high sensitivity of detection was obtained by maintaining the detection potential at 1.23 V. Under optimized conditions, a baseline separation for the two analytes was achieved within 6 min, and the standard curves were linear in the range of 1.0x10(-7) ~ 5.0 x 10(-5) g/mL for LH and 4.0 x 10(-8) ~ 1.0 x 10(-5) g/mL for IF with the detection limits (3sigma) of 6.6 x 10(-8) g/mL for LH and 3.7 x 10(-8) g/mL for IF, respectively. The precisions of intra- and interday measurements for LH and IF were less than 4.21 and 2.61%, respectively. The applicability of the proposed method was illustrated in the determination of LH and IF in rabbit plasma with recoveries between 95.6 and 103.0%.


