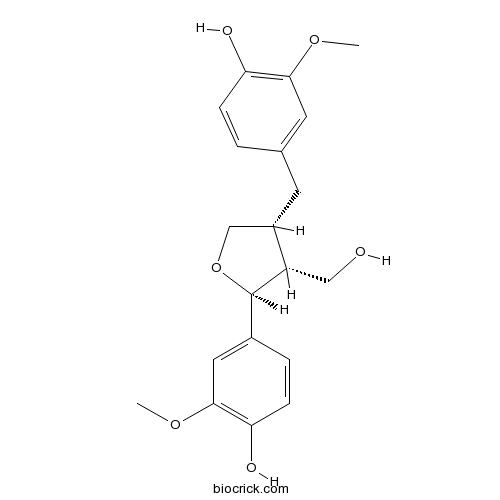
LariciresinolCAS# 27003-73-2 |
- (-)-Lariciresinol
Catalog No.:BCN3418
CAS No.:83327-19-9
- (±)-Lariciresinol
Catalog No.:BCN9662
CAS No.:105367-81-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27003-73-2 | SDF | Download SDF |
| PubChem ID | 332427 | Appearance | Powder |
| Formula | C20H24O6 | M.Wt | 360.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[[(3R,4R,5S)-5-(4-hydroxy-3-methoxyphenyl)-4-(hydroxymethyl)oxolan-3-yl]methyl]-2-methoxyphenol | ||
| SMILES | COC1=C(C=CC(=C1)CC2COC(C2CO)C3=CC(=C(C=C3)O)OC)O | ||
| Standard InChIKey | MHXCIKYXNYCMHY-AUSJPIAWSA-N | ||
| Standard InChI | InChI=1S/C20H24O6/c1-24-18-8-12(3-5-16(18)22)7-14-11-26-20(15(14)10-21)13-4-6-17(23)19(9-13)25-2/h3-6,8-9,14-15,20-23H,7,10-11H2,1-2H3/t14-,15-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dietary lariciresinol can attenuate mammary tumor growth and reduce blood vessel density in human MCF-7 breast cancer xenografts and carcinogen-induced mammary tumors in rats. 2. Lariciresinol is an enterolignan precursor, it possesses fungicidal activities by disrupting the fungal plasma membrane and therapeutic potential as a novel antifungal agent for the treatment of fungal infectious diseases in humans. |
| Targets | VEGFR | Antifection |

Lariciresinol Dilution Calculator

Lariciresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7747 mL | 13.8735 mL | 27.7469 mL | 55.4939 mL | 69.3674 mL |
| 5 mM | 0.5549 mL | 2.7747 mL | 5.5494 mL | 11.0988 mL | 13.8735 mL |
| 10 mM | 0.2775 mL | 1.3873 mL | 2.7747 mL | 5.5494 mL | 6.9367 mL |
| 50 mM | 0.0555 mL | 0.2775 mL | 0.5549 mL | 1.1099 mL | 1.3873 mL |
| 100 mM | 0.0277 mL | 0.1387 mL | 0.2775 mL | 0.5549 mL | 0.6937 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sphingosine-1-phosphate
Catalog No.:BCC7034
CAS No.:26993-30-6
- 16-Acetoxy-7-O-acetylhorminone
Catalog No.:BCN5157
CAS No.:269742-39-4
- Aglaxiflorin D
Catalog No.:BCN6594
CAS No.:269739-78-8
- Timolol Maleate
Catalog No.:BCC4340
CAS No.:26921-17-5
- H-D-Dab-OH.2HCl
Catalog No.:BCC3185
CAS No.:26908-94-1
- Fmoc-Orn(Dde)-OH
Catalog No.:BCC3534
CAS No.:269062-80-8
- Etravirine (TMC125)
Catalog No.:BCC5027
CAS No.:269055-15-4
- 2(-4-Chloro-3-hydroxy-1-butynyl)-5-1,(3-pentadiynyl)thiophene
Catalog No.:BCN1465
CAS No.:26905-70-4
- Oxysophocarpine
Catalog No.:BCN5156
CAS No.:26904-64-3
- 6-Hydroxybenzofuran-2(3H)-one
Catalog No.:BCN5155
CAS No.:2688-49-5
- Isomahanimbine
Catalog No.:BCN3175
CAS No.:26871-46-5
- 1,3-Bis[2-(4-aminophenyl)-2-propyl]benzene
Catalog No.:BCC8419
CAS No.:2687-27-6
- 3,4-Dichloro-Phe-OMe.HCl
Catalog No.:BCC2635
CAS No.:270063-47-3
- 17-Hydroxy-1a,2a-methylenepregna-4,6-diene-3,20-dione acetate
Catalog No.:BCC8442
CAS No.:2701-50-0
- alpha-Hederin
Catalog No.:BCN5159
CAS No.:27013-91-8
- 2'-Methoxykurarinone
Catalog No.:BCN2986
CAS No.:270249-38-2
- H-D-Glu(OMe)-OMe.HCl
Catalog No.:BCC2941
CAS No.:27025-25-8
- Solasurine
Catalog No.:BCN2694
CAS No.:27028-76-8
- Paradol
Catalog No.:BCC1837
CAS No.:27113-22-0
- MMK 1
Catalog No.:BCC6037
CAS No.:271246-66-3
- Thevetin B
Catalog No.:BCN4046
CAS No.:27127-79-3
- 3-Tritylmercapto-Propionicacid
Catalog No.:BCC2846
CAS No.:27144-18-9
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- Thonningianin A
Catalog No.:BCN2774
CAS No.:271579-11-4
Stereoselective syntheses of all stereoisomers of lariciresinol and their plant growth inhibitory activities.[Pubmed:22066904]
J Agric Food Chem. 2011 Dec 28;59(24):13089-95.
All stereoisomers of Lariciresinol were synthesized to examine the effect of stereochemistry on plant growth. Configuration of benzylic 7-positions was constructed through S(N)1 or S(N)2 intramolecular etherification. 8- and 8'-position configurations were established from the starting material except for all cis stereoisomers, the 8-position configurations of which were achieved by employing stereoselective hydroboration. (-)-Lariciresinol and its 7S,8S,8'R stereoisomer inhibited the root growth of Italian ryegrass to 51-55% relative to the negative control, whereas other stereoisomers had less effect. These results demonstrate that the stereochemistry of lignans is one of the important factors influencing their inhibitory activity.
Dietary lariciresinol attenuates mammary tumor growth and reduces blood vessel density in human MCF-7 breast cancer xenografts and carcinogen-induced mammary tumors in rats.[Pubmed:18528864]
Int J Cancer. 2008 Sep 1;123(5):1196-204.
Lariciresinol is a dietary lignan that accounts for a significant portion of the total phytoestrogen intake from Western foods. Recent epidemiological studies suggest that high dietary intake of lignans and Lariciresinol is associated with reduced breast cancer risk. However, no causal relationship between Lariciresinol intake and breast cancer development has been established. In this study, we investigated for the first time the effects and possible mechanisms of action of Lariciresinol on hormone responsive mammary cancer in vivo in dimethylbenz[a]anthracene induced mammary cancer in rats, and in human MCF-7 breast cancer xenografts in athymic mice. For tumor bearing rats, Lariciresinol (3 or 15 mg/kg of body weight) or vehicle was administered p.o. daily for 9 weeks. For E2-maintained ovariectomized athymic mice bearing orthotopic MCF-7 tumors, control diet (AIN-93G) or Lariciresinol containing diet (AIN-93G supplemented with 20 or 100 mg of Lariciresinol/kg of diet) was administered for 5 weeks. In both models, Lariciresinol administration inhibited the tumor growth and tumor angiogenesis. In MCF-7 cells, enterolactone significantly inhibited the E2-stimulated VEGF secretion. Moreover, in MCF-7 xenografts, Lariciresinol administration enhanced tumor cell apoptosis and increased estrogen receptor beta expression. Lariciresinol and its further metabolites secoisoLariciresinol, enterodiol and enterolactone were found in serum of both rats and athymic mice confirming a similar lignan metabolism pattern as in humans. These findings indicate conceivable importance of dietary lignan Lariciresinol in inhibition of breast cancer development.
Structure-plant growth inhibitory activity relationship of lariciresinol.[Pubmed:24274795]
J Agric Food Chem. 2013 Dec 18;61(50):12297-306.
The syntheses of 55 Lariciresinol derivatives containing derivatives on the 9-position and an aryl group at both 7- and 7'-positions were successful to examine the effect of structure of (-)-Lariciresinol (1) on plant growth regulatory activity. (-)-(7R,8R,8'S)-9-DehydroxyLariciresinol 9 showed activity 2-fold more potent than that of natural (-)-Lariciresinol (1) and -95% growth inhibitory activity to negative control against rye grass root at 1 mM. The derivatives bearing hydrophobic and smaller groups at the 9-position showed higher activity. The importance of 4- and 4'-hydroxy groups and 3- and 3'-small hydrophobic groups on 7- and 7'-phenyl groups for higher activity was also suggested.
Antifungal activity of lariciresinol derived from Sambucus williamsii and their membrane-active mechanisms in Candida albicans.[Pubmed:21679690]
Biochem Biophys Res Commun. 2011 Jul 8;410(3):489-93.
Lariciresinol is an enterolignan precursor isolated from the herb Sambucus williamsii, a folk medicinal plant used for its therapeutic properties. In this study, the antifungal properties and mode of action of Lariciresinol were investigated. Lariciresinol displays potent antifungal properties against several human pathogenic fungal strains without hemolytic effects on human erythrocytes. To understand the antifungal mechanism of action of Lariciresinol, the membrane interactions of Lariciresinol were examined. Fluorescence analysis using the membrane probe 3,3'-diethylthio-dicarbocyanine iodide (DiSC(3)-5) and 1,6-diphenyl-1,3,5-hexatriene (DPH), as well as a flow cytometric analysis with propidium iodide (PI), a membrane-impermeable dye, indicated that Lariciresinol was associated with lipid bilayers and induced membrane permeabilization. Therefore, the present study suggests that Lariciresinol possesses fungicidal activities by disrupting the fungal plasma membrane and therapeutic potential as a novel antifungal agent for the treatment of fungal infectious diseases in humans.


