ParadolActive flavor constituent of the seeds of Guinea pepper CAS# 27113-22-0 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27113-22-0 | SDF | Download SDF |
| PubChem ID | 94378 | Appearance | Powder |
| Formula | C17H26O3 | M.Wt | 278.39 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | [6]-Gingerone; [6]-Paradol | ||
| Solubility | DMSO : ≥ 140 mg/mL (502.89 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1-(4-hydroxy-3-methoxyphenyl)decan-3-one | ||
| SMILES | CCCCCCCC(=O)CCC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | CZNLTCTYLMYLHL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H26O3/c1-3-4-5-6-7-8-15(18)11-9-14-10-12-16(19)17(13-14)20-2/h10,12-13,19H,3-9,11H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (3)-Paradol has antimicrobial activity. |
| Targets | Antifection |

Paradol Dilution Calculator

Paradol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5921 mL | 17.9604 mL | 35.9208 mL | 71.8417 mL | 89.8021 mL |
| 5 mM | 0.7184 mL | 3.5921 mL | 7.1842 mL | 14.3683 mL | 17.9604 mL |
| 10 mM | 0.3592 mL | 1.796 mL | 3.5921 mL | 7.1842 mL | 8.9802 mL |
| 50 mM | 0.0718 mL | 0.3592 mL | 0.7184 mL | 1.4368 mL | 1.796 mL |
| 100 mM | 0.0359 mL | 0.1796 mL | 0.3592 mL | 0.7184 mL | 0.898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
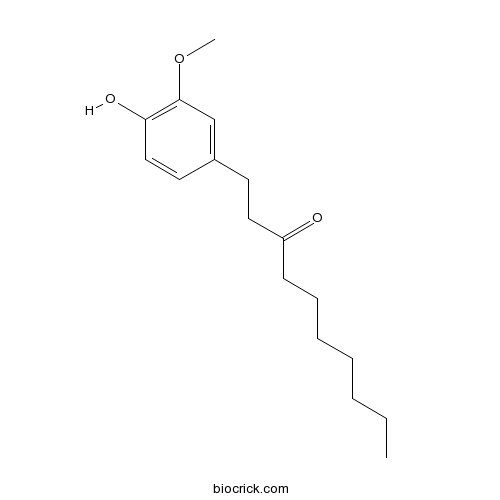
Paradol is the active flavor constituent of the seeds of Guinea pepper (Aframomum melegueta). The seed is also known as Grains of paradise. Paradol has been found to have antioxidative and antitumor promoting effects.
- Solasurine
Catalog No.:BCN2694
CAS No.:27028-76-8
- H-D-Glu(OMe)-OMe.HCl
Catalog No.:BCC2941
CAS No.:27025-25-8
- 2'-Methoxykurarinone
Catalog No.:BCN2986
CAS No.:270249-38-2
- alpha-Hederin
Catalog No.:BCN5159
CAS No.:27013-91-8
- 17-Hydroxy-1a,2a-methylenepregna-4,6-diene-3,20-dione acetate
Catalog No.:BCC8442
CAS No.:2701-50-0
- 3,4-Dichloro-Phe-OMe.HCl
Catalog No.:BCC2635
CAS No.:270063-47-3
- Lariciresinol
Catalog No.:BCN5158
CAS No.:27003-73-2
- Sphingosine-1-phosphate
Catalog No.:BCC7034
CAS No.:26993-30-6
- 16-Acetoxy-7-O-acetylhorminone
Catalog No.:BCN5157
CAS No.:269742-39-4
- Aglaxiflorin D
Catalog No.:BCN6594
CAS No.:269739-78-8
- Timolol Maleate
Catalog No.:BCC4340
CAS No.:26921-17-5
- H-D-Dab-OH.2HCl
Catalog No.:BCC3185
CAS No.:26908-94-1
- MMK 1
Catalog No.:BCC6037
CAS No.:271246-66-3
- Thevetin B
Catalog No.:BCN4046
CAS No.:27127-79-3
- 3-Tritylmercapto-Propionicacid
Catalog No.:BCC2846
CAS No.:27144-18-9
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- Thonningianin A
Catalog No.:BCN2774
CAS No.:271579-11-4
- LY 393558
Catalog No.:BCC7660
CAS No.:271780-64-4
- FH1(BRD-K4477)
Catalog No.:BCC5341
CAS No.:2719-05-3
- 2-Acetamidothiazole
Catalog No.:BCC8509
CAS No.:2719-23-5
- Ampelopsin
Catalog No.:BCN5160
CAS No.:27200-12-0
- Bis[4-(2-hydroxyethoxy)phenyl] sulfone
Catalog No.:BCC8888
CAS No.:27205-03-4
- Polydatin
Catalog No.:BCN5949
CAS No.:27208-80-6
- Miltirone
Catalog No.:BCN5356
CAS No.:27210-57-7
Neuroprotective effect of 6-paradol in focal cerebral ischemia involves the attenuation of neuroinflammatory responses in activated microglia.[Pubmed:25789481]
PLoS One. 2015 Mar 19;10(3):e0120203.
Paradols are non-pungent and biotransformed metabolites of shogaols and reduce inflammatory responses as well as oxidative stress as shogaols. Recently, shogaol has been noted to possess therapeutic potential against several central nervous system (CNS) disorders, including cerebral ischemia, by reducing neuroinflammation in microglia. Therefore, Paradol could be used to improve neuroinflammation-associated CNS disorders. Here, we synthesized Paradol derivatives (2- to 10-Paradols). Through the initial screening for anti-inflammatory activities using lipopolysaccharide (LPS)-stimulated BV2 microglia, 6-Paradol was chosen to be the most effective compound without cytotoxicity. Pretreatment with 6-Paradol reduced neuroinflammatory responses in LPS-stimulated BV2 microglia by a concentration-dependent manner, which includes reduced NO production by inhibiting iNOS upregulation and lowered secretion of proinflammatory cytokines (IL-6 and TNF-alpha). To pursue whether the beneficial in vitro effects of 6-Paradol leads towards in vivo therapeutic effects on transient focal cerebral ischemia characterized by neuroinflammation, we employed middle cerebral artery occlusion (MCAO)/reperfusion (M/R). Administration of 6-Paradol immediately after reperfusion significantly reduced brain damage in M/R-challenged mice as assessed by brain infarction, neurological deficit, and neural cell survival and death. Furthermore, as observed in cultured microglia, 6-Paradol administration markedly reduced neuroinflammation in M/R-challenged brains by attenuating microglial activation and reducing the number of cells expressing iNOS and TNF-alpha, both of which are known to be produced in microglia following M/R challenge. Collectively, this study provides evidences that 6-Paradol effectively protects brain after cerebral ischemia, likely by attenuating neuroinflammation in microglia, suggesting it as a potential therapeutic agent to treat cerebral ischemia.
6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice.[Pubmed:28106738]
Int J Mol Sci. 2017 Jan 17;18(1). pii: ijms18010168.
The anti-diabetic activity of ginger powder (Zingiber officinale) has been recently promoted, with the recommendation to be included as one of the dietary supplements for diabetic patients. However, previous studies presented different results, which may be caused by degradation and metabolic changes of ginger components, gingerols, shogaols and Paradols. Therefore, we prepared 10 ginger active components, namely 6-, 8-, 10-Paradols, 6-, 8-, 10-shogaols, 6-, 8-, 10-gingerols and zingerone, and evaluated their anti-hyperglycemic activity. Among the tested compounds, 6-Paradol and 6-shogaol showed potent activity in stimulating glucose utilization by 3T3-L1 adipocytes and C2C12 myotubes. The effects were attributed to the increase in 5' adenosine monophosphate-activated protein kinase (AMPK) phosphorylation in 3T3-L1 adipocytes. 6-Paradol, the major metabolite of 6-shogaol, was utilized in an in vivo assay and significantly reduced blood glucose, cholesterol and body weight in high-fat diet-fed mice.
Effects of 6-paradol, an unsaturated ketone from gingers, on cytochrome P450-mediated drug metabolism.[Pubmed:28274629]
Bioorg Med Chem Lett. 2017 Apr 15;27(8):1826-1830.
Paradols are unsaturated ketones produced by biotransformation of shogaols in gingers. Among them, 6-Paradol has been investigated as a new drug candidate due to its anti-inflammatory, apoptotic, and neuroprotective activities. In this study, the inhibitory effects of 6-Paradol on the activities of cytochrome P450 (CYP) enzymes were investigated with human liver microsomes and recombinant CYP isozymes. 6-Paradol showed concentration-dependent inhibitory effects on CYP1A2, CYP2B6, CYP2C8, CYP2C9, and CYP2C19 isozymes, with IC50 values ranging from 3.8 to 21.4microM in recombinant CYP isozymes. However, the inhibition was not potentiated following pre-incubation, indicating that 6-Paradol is not a mechanism-based inhibitor. These results suggest that pharmacokinetic drug-drug interactions might occur with 6-Paradol, which must be considered in the process of new drug development.
Pharmacokinetics of Paradol Analogues Orally Administered to Rats.[Pubmed:26868188]
J Agric Food Chem. 2016 Mar 9;64(9):1932-7.
The kinetics parameters of Paradols with different acyl chain lengths have been evaluated to determine their antiobesity site of action. Rats were orally administered olive oil containing 0-, 6-, 8-, or 12-Paradol, and blood samples were collected at different time points. The concentrations of the Paradols in the plasma were analyzed both with and without beta-glucuronidase treatment. The area under the plasma concentration-time curve from 0 to 24 h (AUC(0-24h)) of the parent compounds decreased with increasing acyl chain length. Whereas 12-Paradol showed the largest AUC(0-24h) with the longest time to reach its maximum plasma concentration of all of the compounds tested, the AUC(0-24h) values of the metabolites decreased with increasing acyl chain length. These results indicate that increasing acyl chain length leads to a decrease in the absorption of Paradols via the intestinal tract, the wall of which was estimated to be their antiobesity site of action.


