MiltironeCAS# 27210-57-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27210-57-7 | SDF | Download SDF |
| PubChem ID | 160142 | Appearance | Red powder |
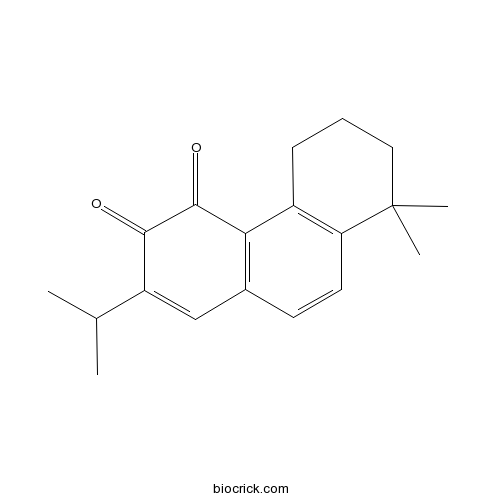
| Formula | C19H22O2 | M.Wt | 282.38 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 8,8-dimethyl-2-propan-2-yl-6,7-dihydro-5H-phenanthrene-3,4-dione | ||
| SMILES | CC(C)C1=CC2=C(C3=C(C=C2)C(CCC3)(C)C)C(=O)C1=O | ||
| Standard InChIKey | FEFAIBOZOKSLJR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H22O2/c1-11(2)14-10-12-7-8-15-13(6-5-9-19(15,3)4)16(12)18(21)17(14)20/h7-8,10-11H,5-6,9H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Miltirone is a CYPs inhibition, it has been characterized as a low-affinity ligand for central benzodiazepine receptors, it might ameliorate the symptoms associated with discontinuation of long-term administration of ethanol or of other positive modulators of the GABA A receptor. Miltirone possesses significant anticancer, antibacterial, antioxidant, and anti-inflammatory activities, the hepatocyte metabolism is the major route of clearance for miltirone. Miltirone is collateral sensitive in multidrug-resistant P-gp-overexpressing cells, induces G2/M arrest, and triggeres apoptosis via ROS-generated breakdown of MMP and DNA damage. Miltirone has antiprotozoal activity against T. brucei rhodesiense STIB 900. |
| Targets | P-gp | PARP | ROS | IkB | MMP(e.g.TIMP) | p21 | P450 (e.g. CYP17) | GABA Receptor | Calcium Channel | Bcl-2/Bax | Antifection | IKK |
| In vitro | Miltirone Induces G2/M Cell Cycle Arrest and Apoptosis in CCRF-CEM Acute Lymphoblastic Leukemia Cells.[Pubmed: 26035463]J Nat Prod. 2015 Jun 26;78(6):1339-47.Miltirone (1) is a diterpene quinone extracted from a well-known Chinese traditional herb (Salvia miltiorrhiza). We investigated the cytotoxic effects of Miltirone toward sensitive and multidrug-resistant acute lymphoblastic leukemia cell lines.
Antiplasmodial and antitrypanosomal activity of tanshinone-type diterpenoids from Salvia miltiorrhiza.[Pubmed: 21412700 ]Planta Med. 2011 Sep;77(14):1594-6.In a medium throughput screen of 880 plant and fungal extracts for antiprotozoal activity, a dichloromethane extract of Salvia miltiorrhiza roots was active against both Trypanosoma brucei rhodesiense and Plasmodium falciparum.
|
| In vivo | Identification of miltirone as active ingredient of Salvia miltiorrhiza responsible for the reducing effect of root extracts on alcohol intake in rats.[Pubmed: 16634843]Alcohol Clin Exp Res. 2006 May;30(5):754-62.Previous work found that extracts from the roots of Salvia miltiorrhiza, a Chinese medicinal herb, reduced alcohol intake in selectively bred Sardinian alcohol-preferring (sP) rats. The present study was designed to evaluate whether Miltirone, one of the possible active constituents of S. miltiorrhiza, might be responsible for the reducing effect of the extracts on alcohol intake.
|
| Kinase Assay | Inhibition by miltirone of up-regulation of GABAA receptor alpha4 subunit mRNA by ethanol withdrawal in hippocampal neurons.[Pubmed: 15212961]Enzyme kinetic and molecular docking studies for the inhibitions of miltirone on major human cytochrome P450 isozymes.[Pubmed: 23102508]Phytomedicine. 2013 Feb 15;20(3-4):367-74.Previous studies have shown that major tanshinones isolated from Danshen (Salvia miltiorrhiza) inhibited human and rat CYP450 enzymes-mediated metabolism of model probe substrates, with potential in causing herb-drug interactions. Miltirone, another abietane type-diterpene quinone isolated from Danshen, has been reported for its anti-oxidative, anxiolytic and anti-cancer effects.
Eur J Pharmacol. 2004 Jun 28;494(2-3):83-90.Miltirone, a tanshinone isolated from the root of Salvia miltiorrhiza, has been characterized as a low-affinity ligand for central benzodiazepine receptors.
|
| Cell Research | Miltirone exhibits antileukemic activity by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction pathways.[Pubmed: 26848099 ]Sci Rep. 2016 Feb 5;6:20585.In this study, we investigated the effects of Miltirone in human leukemia cell lines, primary leukemia cells, and nude mice U937 xenograft.
|
| Structure Identification | J Pharm Biomed Anal. 2015 Mar 25;107:473-9.Metabolic profile of miltirone in rats by high performance liquid chromatography/quadrupole time-of-flight mass spectrometry.[Pubmed: 25679091]Miltirone is one of the bioactive diterpene quinones isolated from Salvia miltiorrhiza Bunge. This compound has been found to possess significant anticancer, antibacterial, antioxidant, and anti-inflammatory activities.
However, the metabolic fate of Miltirone remains unknown. In order to explore whether Miltirone is extensively metabolized, we investigated the metabolites of Miltirone in plasma, bile, urine, and feces samples following oral and intravenous administration to the rats. |

Miltirone Dilution Calculator

Miltirone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5413 mL | 17.7066 mL | 35.4133 mL | 70.8265 mL | 88.5332 mL |
| 5 mM | 0.7083 mL | 3.5413 mL | 7.0827 mL | 14.1653 mL | 17.7066 mL |
| 10 mM | 0.3541 mL | 1.7707 mL | 3.5413 mL | 7.0827 mL | 8.8533 mL |
| 50 mM | 0.0708 mL | 0.3541 mL | 0.7083 mL | 1.4165 mL | 1.7707 mL |
| 100 mM | 0.0354 mL | 0.1771 mL | 0.3541 mL | 0.7083 mL | 0.8853 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Polydatin
Catalog No.:BCN5949
CAS No.:27208-80-6
- Bis[4-(2-hydroxyethoxy)phenyl] sulfone
Catalog No.:BCC8888
CAS No.:27205-03-4
- Ampelopsin
Catalog No.:BCN5160
CAS No.:27200-12-0
- 2-Acetamidothiazole
Catalog No.:BCC8509
CAS No.:2719-23-5
- FH1(BRD-K4477)
Catalog No.:BCC5341
CAS No.:2719-05-3
- LY 393558
Catalog No.:BCC7660
CAS No.:271780-64-4
- Thonningianin A
Catalog No.:BCN2774
CAS No.:271579-11-4
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- 3-Tritylmercapto-Propionicacid
Catalog No.:BCC2846
CAS No.:27144-18-9
- Thevetin B
Catalog No.:BCN4046
CAS No.:27127-79-3
- MMK 1
Catalog No.:BCC6037
CAS No.:271246-66-3
- Paradol
Catalog No.:BCC1837
CAS No.:27113-22-0
- Pedunsaponin C
Catalog No.:BCN8193
CAS No.:272120-53-3
- Decursidate
Catalog No.:BCN4044
CAS No.:272122-56-2
- Cyanidin 3-Arabinoside
Catalog No.:BCC8157
CAS No.:27214-72-8
- Neoandrographolide
Catalog No.:BCN4657
CAS No.:27215-14-1
- N-(2,6-Dimethylphenyl)-2-piperidinecarboxamide
Catalog No.:BCC9051
CAS No.:27262-40-4
- Levobupivacaine HCl
Catalog No.:BCC4675
CAS No.:27262-48-2
- Phyllostine
Catalog No.:BCN4773
CAS No.:27270-89-9
- ICI 63197
Catalog No.:BCC7188
CAS No.:27277-00-5
- Tirapazamine
Catalog No.:BCC5184
CAS No.:27314-97-2
- 5-Formyl-2-furylboronic acid
Catalog No.:BCC8748
CAS No.:27329-70-0
- Precyasterone
Catalog No.:BCN2754
CAS No.:27335-85-9
- VX-765
Catalog No.:BCC3648
CAS No.:273404-37-8
Antiplasmodial and antitrypanosomal activity of tanshinone-type diterpenoids from Salvia miltiorrhiza.[Pubmed:21412700]
Planta Med. 2011 Sep;77(14):1594-6.
In a medium throughput screen of 880 plant and fungal extracts for antiprotozoal activity, a dichloromethane extract of Salvia miltiorrhiza roots was active against both Trypanosoma brucei rhodesiense and Plasmodium falciparum. With HPLC-based activity profiling in combination with on- and off-line spectroscopic methods (PDA, -MS (n), HR-MS, microprobe NMR), the active compounds were identified as tanshinone-type diterpenoids. Subsequent isolation and structure elucidation yielded the known substances Miltirone (1), tanshinone II a (2), 1,2 dihydrotanshinquinone (3), methylenetanshinquinone (4), 1-oxoMiltirone (5), 11-hydroxymiltiodiol (6), tanshinone I (7), methyltanshinonate (8), and cryptotanshinone (9). The IC(5)(0)s of the compounds were determined against the two parasites and rat myoblast (L6) cells. They ranged from 4.1 microM to over 30 microM against P. falciparum K1 strain with selectivity indices (SI) from 0.3 to 1.9. IC(5)(0)s against T. brucei rhodesiense STIB 900 were from 0.5 microM (1, 4) to over 30 microM, and 4 showed the greatest selective activity with an SI of 24.
Metabolic profile of miltirone in rats by high performance liquid chromatography/quadrupole time-of-flight mass spectrometry.[Pubmed:25679091]
J Pharm Biomed Anal. 2015 Mar 25;107:473-9.
Miltirone is one of the bioactive diterpene quinones isolated from Salvia miltiorrhiza Bunge. This compound has been found to possess significant anticancer, antibacterial, antioxidant, and anti-inflammatory activities. However, the metabolic fate of Miltirone remains unknown. In order to explore whether Miltirone is extensively metabolized, we investigated the metabolites of Miltirone in plasma, bile, urine, and feces samples following oral and intravenous administration to the rats. By using high performance liquid chromatography/quadrupole time-of-flight mass spectrometry (HPLC/Q-TOF-MS) coupled with mass detect filter (MDF) method, a total of 15 metabolites were identified from the biosamples. Both phase I and phase II metabolites were observed in the metabolic profile and the metabolic pathways involved in reduction, oxidation, monohydroxylation, dihydroxylation, glucuronidation and sulfation. The results indicated that hepatocyte metabolism was the major route of clearance for the parent compound. The present study provided valuable information for better understanding of the efficacy and safety of Miltirone.
Inhibition by miltirone of up-regulation of GABAA receptor alpha4 subunit mRNA by ethanol withdrawal in hippocampal neurons.[Pubmed:15212961]
Eur J Pharmacol. 2004 Jun 28;494(2-3):83-90.
Miltirone, a tanshinone isolated from the root of Salvia miltiorrhiza, has been characterized as a low-affinity ligand for central benzodiazepine receptors. We have now shown that this compound bound with low affinity (micromolar range) to central benzodiazepine recognition sites but did not interact with peripheral benzodiazepine receptors. It failed to potentiate Cl(-) currents induced by gamma-aminobutyric acid (GABA) both in Xenopus oocytes expressing recombinant human GABA(A) receptors and in cultured rat hippocampal pyramidal cells, but it inhibited the ability of diazepam to potentiate the effect of GABA in these systems. Miltirone (1-10 microM) also partially inhibited the increase in the abundance of the mRNA for the alpha(4) subunit of the GABA(A) receptor induced by ethanol withdrawal in cultured hippocampal neurons. These results suggest that Miltirone might ameliorate the symptoms associated with discontinuation of long-term administration of ethanol or of other positive modulators of the GABA(A) receptor.
Enzyme kinetic and molecular docking studies for the inhibitions of miltirone on major human cytochrome P450 isozymes.[Pubmed:23102508]
Phytomedicine. 2013 Feb 15;20(3-4):367-74.
Previous studies have shown that major tanshinones isolated from Danshen (Salvia miltiorrhiza) inhibited human and rat CYP450 enzymes-mediated metabolism of model probe substrates, with potential in causing herb-drug interactions. Miltirone, another abietane type-diterpene quinone isolated from Danshen, has been reported for its anti-oxidative, anxiolytic and anti-cancer effects. The aim of this study was to study the effect of Miltirone on the metabolism of model probe substrates of CYP1A2, 2C9, 2D6 and 3A4 in pooled human liver microsomes. Miltirone showed moderate inhibition on CYP1A2 (IC(50)=1.73 muM) and CYP2C9 (IC(50)=8.61 muM), and weak inhibition on CYP2D6 (IC(50)=30.20 muM) and CYP3A4 (IC(50)=33.88 muM). Enzyme kinetic studies showed that Miltirone competitively inhibited CYP2C9 (K(i)=1.48 muM), and displayed mixed type inhibitions on CYP1A2, CYP2D6 and CYP3A4 with K(i) values of 3.17 muM, 24.25 muM and 35.09 muM, respectively. Molecular docking study further confirmed the ligand-binding conformations of Miltirone in the active sites of these human CYP450 isoforms, and provided some information on structure-activity relationships for the CYPs inhibition by tanshinones. Taken together, CYPs inhibitions of Miltirone were weaker than dihydrotanshinone, but stronger than cryptotanshinone, tanshinone I and tanshinone IIA.
Identification of miltirone as active ingredient of Salvia miltiorrhiza responsible for the reducing effect of root extracts on alcohol intake in rats.[Pubmed:16634843]
Alcohol Clin Exp Res. 2006 May;30(5):754-62.
BACKGROUND: Previous work found that extracts from the roots of Salvia miltiorrhiza, a Chinese medicinal herb, reduced alcohol intake in selectively bred Sardinian alcohol-preferring (sP) rats. The present study was designed to evaluate whether Miltirone, one of the possible active constituents of S. miltiorrhiza, might be responsible for the reducing effect of the extracts on alcohol intake. METHODS: An initial experiment assessed the effect of 100 mg/kg (intragastric, i.g.) of 4 extracts of S. miltiorrhiza, differing in Miltirone content (0, 2, 3, and 7%, respectively), on alcohol intake in alcohol-experienced sP rats exposed to the 2-bottle "alcohol (10%, volume in volume) versus water" choice regimen. Subsequently, the effect of pure Miltirone (2.5-10 mg/kg, i.g., i.e., a dose range comparable to its content in the effective doses of the active extracts) on acquisition and maintenance of alcohol-drinking behavior was evaluated in alcohol-naive and alcohol-experienced sP rats exposed to the 2-bottle choice regimen. The effect of Miltirone (10 mg/kg, i.g.) on blood alcohol levels was assessed after the i.g. and intraperitoneal (i.p.) administration of alcohol. Finally, the effect of Miltirone (30-100 mg/kg, i.g.) on the severity of alcohol withdrawal syndrome was evaluated in Wistar rats made physically dependent on alcohol by the repeated administration of intoxicating doses of alcohol. RESULTS: The reducing effect of 4 different extracts of S. miltiorrhiza on alcohol intake was positively and significantly correlated with their Miltirone content. Pure Miltirone reduced alcohol intake in alcohol-experienced rats and delayed acquisition of alcohol-drinking behavior in alcohol-naive rats. Similar to S. miltiorrhiza extracts, Miltirone markedly reduced blood alcohol levels when alcohol was administered i.g. but not i.p., suggesting that Miltirone hampered alcohol absorption from the gastrointestinal system. Finally, Miltirone failed to affect the severity of alcohol withdrawal syndrome in alcohol-dependent rats. CONCLUSIONS: The results of the present study suggest that Miltirone is the likely active constituent of S. miltiorrhiza responsible for the reducing effect of its extracts on alcohol intake in different experimental models of excessive alcohol consumption.
Miltirone exhibits antileukemic activity by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction pathways.[Pubmed:26848099]
Sci Rep. 2016 Feb 5;6:20585.
In this study, we investigated the effects of Miltirone in human leukemia cell lines, primary leukemia cells, and nude mice U937 xenograft. Treatment of cells with Miltirone resulted in apoptosis, mitochondria membrane potential (MMP) collapses, increase of Bax/Bcl-2 ratio, and cytochrome c release. Miltirone triggered the endoplasmic reticulum (ER) stress identified through several key molecules of the unfolded protein response, including phosphorylated PERK, eIF2a, GRP78, GRP94, and caspase-12. Miltrone treatment also resulted in the release of Ca(2+) from the ER stores and mitochondrial Ca(2+) loading in the cells. Further research revealed that Miltirone resulted in dose-dependent decrease in complex III activity and elevated reactive oxygen species (ROS) production in these cells. Miltirone-induced apoptosis, dissipation of MMP and ER stress were dramatically blocked by pretreatment with antioxidant N-acetylcysteine (NAC). In contrast, treatment with ER stress inhibitor TUDCA significantly attenuated Miltirone-induced ROS and apoptosis in leukemia cells. Moreover, our in vivo findings showed that administration of Miltirone markedly inhibited tumor growth and induced apoptosis in U937 xenograft model with low systemic toxicity. Taken together, these findings indicate that Miltirone may exert its antileukemic activity by inducing apoptosis through a ROS-dependent destructive cycle involving ER stress and mitochondrial dysfunction.
Miltirone Induces G2/M Cell Cycle Arrest and Apoptosis in CCRF-CEM Acute Lymphoblastic Leukemia Cells.[Pubmed:26035463]
J Nat Prod. 2015 Jun 26;78(6):1339-47.
Miltirone (1) is a diterpene quinone extracted from a well-known Chinese traditional herb (Salvia miltiorrhiza). We investigated the cytotoxic effects of Miltirone toward sensitive and multidrug-resistant acute lymphoblastic leukemia cell lines. Miltirone inhibited multidrug-resistant P-glycoprotein (P-gp)-overexpressing CEM/ADR5000 cells better than drug-sensitive CCRF-CEM wild-type cells, a phenomenon termed collateral sensitivity. Flow cytometric analyses revealed that Miltirone induced G2/M arrest and apoptosis. Furthermore, Miltirone stimulated reactive oxygen species (ROS) generation and mitochondrial membrane potential (MMP) disruption, which in turn induced DNA damage and activation of caspases and poly ADP-ribose polymerase (PARP). Downregulation of CCNB1 (cyclin B1) and CDC2 mRNA and upregulation of CDKN1A (p21) mRNA were in accord with Miltirone-induced G2/M arrest. Moreover, Miltirone decreased cell adherence to fibronectin. Molecular docking revealed that Miltirone bound to the ATP-binding site of IKK-beta. In conclusion, Miltirone was collateral sensitive in multidrug-resistant P-gp-overexpressing cells, induced G2/M arrest, and triggered apoptosis via ROS-generated breakdown of MMP and DNA damage. Therefore, Miltirone may be a promising candidate for cancer chemotherapy.


