ICI 63197PDE4 inhibitor CAS# 27277-00-5 |
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- IWR-1-endo
Catalog No.:BCC5102
CAS No.:1127442-82-3
- CCT 031374 hydrobromide
Catalog No.:BCC6258
CAS No.:1219184-91-4
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- iCRT 14
Catalog No.:BCC5401
CAS No.:677331-12-3
- IWP-2
Catalog No.:BCC1665
CAS No.:686770-61-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27277-00-5 | SDF | Download SDF |
| PubChem ID | 62824 | Appearance | Powder |
| Formula | C9H13N5O | M.Wt | 207.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
| Chemical Name | 2-amino-6-methyl-4-propyl-[1,2,4]triazolo[1,5-a]pyrimidin-5-one | ||
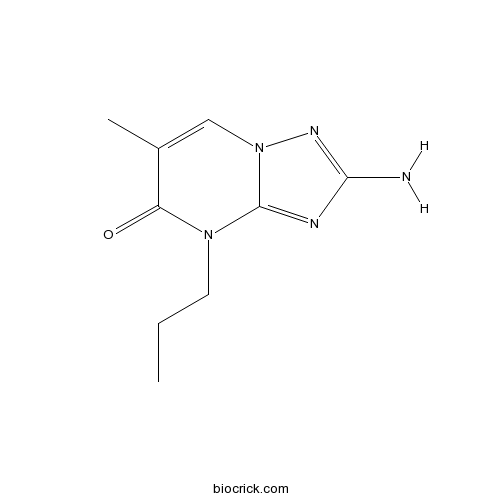
| SMILES | CCCN1C(=O)C(=CN2C1=NC(=N2)N)C | ||
| Standard InChIKey | UQDVRVNMIJAGRK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H13N5O/c1-3-4-13-7(15)6(2)5-14-9(13)11-8(10)12-14/h5H,3-4H2,1-2H3,(H2,10,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent phosphodiesterase (PDE) 4 inhibitor (IC50 = 35 nM for inhibition of [3H]-rolipram binding to rat brain). Antidepressant following systemic administration in vivo. |

ICI 63197 Dilution Calculator

ICI 63197 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8256 mL | 24.1278 mL | 48.2556 mL | 96.5111 mL | 120.6389 mL |
| 5 mM | 0.9651 mL | 4.8256 mL | 9.6511 mL | 19.3022 mL | 24.1278 mL |
| 10 mM | 0.4826 mL | 2.4128 mL | 4.8256 mL | 9.6511 mL | 12.0639 mL |
| 50 mM | 0.0965 mL | 0.4826 mL | 0.9651 mL | 1.9302 mL | 2.4128 mL |
| 100 mM | 0.0483 mL | 0.2413 mL | 0.4826 mL | 0.9651 mL | 1.2064 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Phyllostine
Catalog No.:BCN4773
CAS No.:27270-89-9
- Levobupivacaine HCl
Catalog No.:BCC4675
CAS No.:27262-48-2
- N-(2,6-Dimethylphenyl)-2-piperidinecarboxamide
Catalog No.:BCC9051
CAS No.:27262-40-4
- Neoandrographolide
Catalog No.:BCN4657
CAS No.:27215-14-1
- Cyanidin 3-Arabinoside
Catalog No.:BCC8157
CAS No.:27214-72-8
- Decursidate
Catalog No.:BCN4044
CAS No.:272122-56-2
- Pedunsaponin C
Catalog No.:BCN8193
CAS No.:272120-53-3
- Miltirone
Catalog No.:BCN5356
CAS No.:27210-57-7
- Polydatin
Catalog No.:BCN5949
CAS No.:27208-80-6
- Bis[4-(2-hydroxyethoxy)phenyl] sulfone
Catalog No.:BCC8888
CAS No.:27205-03-4
- Ampelopsin
Catalog No.:BCN5160
CAS No.:27200-12-0
- 2-Acetamidothiazole
Catalog No.:BCC8509
CAS No.:2719-23-5
- Tirapazamine
Catalog No.:BCC5184
CAS No.:27314-97-2
- 5-Formyl-2-furylboronic acid
Catalog No.:BCC8748
CAS No.:27329-70-0
- Precyasterone
Catalog No.:BCN2754
CAS No.:27335-85-9
- VX-765
Catalog No.:BCC3648
CAS No.:273404-37-8
- 4'-Hydroxy-7-methoxyflavan
Catalog No.:BCN3497
CAS No.:27348-54-5
- threo-Guaiacylglycerol
Catalog No.:BCN5161
CAS No.:27391-16-8
- Picroside I
Catalog No.:BCN6322
CAS No.:27409-30-9
- Coumarin 7
Catalog No.:BCC8920
CAS No.:27425-55-4
- 5-Methoxyisatin
Catalog No.:BCC8098
CAS No.:39755-95-8
- Xanthohumol D
Catalog No.:BCN5162
CAS No.:274675-25-1
- Neocryptotanshinone II
Catalog No.:BCN3138
CAS No.:27468-20-8
- Ticagrelor
Catalog No.:BCC4975
CAS No.:274693-27-5
Tamoxifen and ICI 182,780 activate hypothalamic G protein-coupled estrogen receptor 1 to rapidly facilitate lordosis in female rats.[Pubmed:28063803]
Horm Behav. 2017 Mar;89:98-103.
In the female rat, sexual receptivity (lordosis) can be facilitated by sequential activation of estrogen receptor (ER) alpha and G protein-coupled estrogen receptor 1 (GPER) by estradiol. In the estradiol benzoate (EB) primed ovariectomized (OVX) rat, EB initially binds to ERalpha in the plasma membrane that complexes with and transactivates metabotropic glutamate receptor 1a to activate beta-endorphin neurons in the arcuate nucleus of the hypothalamus (ARH) that project to the medial preoptic nucleus (MPN). This activates MPN mu-opioid receptors (MOP), inhibiting lordosis. Infusion of non-esterified 17beta-estradiol into the ARH rapidly reduces MPN MOP activation and facilitates lordosis via GPER. Tamoxifen (TAM) and ICI 182,780 (ICI) are selective estrogen receptor modulators that activate GPER. Therefore, we tested the hypothesis that TAM and ICI rapidly facilitate lordosis via activation of GPER in the ARH. Our first experiment demonstrated that injection of TAM intraperitoneal, or ICI into the lateral ventricle, deactivated MPN MOP and facilitated lordosis in EB-primed rats. We then tested whether TAM and ICI were acting rapidly through a GPER dependent pathway in the ARH. In EB-primed rats, ARH infusion of either TAM or ICI facilitated lordosis and reduced MPN MOP activation within 30min compared to controls. These effects were blocked by pretreatment with the GPER antagonist, G15. Our findings demonstrate that TAM and ICI deactivate MPN MOP and facilitate lordosis in a GPER dependent manner. Thus, TAM and ICI may activate GPER in the CNS to produce estrogenic actions in neural circuits that modulate physiology and behavior.
ICI-RS 2015-Is a better understanding of sleep the key in managing nocturia?[Pubmed:27653805]
Neurourol Urodyn. 2018 Sep;37(7):2048-2052.
AIMS: Nocturia, or waking up at night to void, is a highly prevalent and bothersome lower urinary tract symptom. However, the applied treatment modalities do not improve symptoms in about half of the patients. The aim of this report is to generate new ideas for future nocturia research, with special emphasis on the role of sleep physiology and sleep disorders. METHODS: The following is a report of the presentations and subsequent discussion of the Nocturia Think Tank session at the annual meeting of the International Consultation on Incontinence Research Society (ICI-RS), which took place in September 2015 in Bristol. General information about the organization of the ICI-RS meeting can be found on the website "www.ici-rs.org." An overview of challenges within the existing evidence, future research ideas, and results of research with regard to nocturia and sleep were presented. RESULTS AND CONCLUSION: In order to optimize the management of nocturia and nocturnal polyuria (NP), future research has to focus on the development of unambiguous terminology regarding nocturia and NP, the role of renal function profiles and simplified frequency volume charts as guidance of individualized therapy and the role of sleep disorders such as periodic limb movements during sleep and habitual voiding as a response to awakening. Neurourol. Urodynam. 37:2048-2052, 2018. (c) 2016 Wiley Periodicals, Inc.
Inhibitor binding to type 4 phosphodiesterase (PDE4) assessed using [3H]piclamilast and [3H]rolipram.[Pubmed:12704225]
J Pharmacol Exp Ther. 2003 May;305(2):565-72.
Piclamilast is a type 4 phosphodiesterase (PDE4) inhibitor with equal affinity for the high-affinity rolipram binding site (HARBS) and low-affinity rolipram binding site (LARBS). The binding of [(3)H]piclamilast to preparations of rat brain and peripheral tissue was investigated and compared with that of [(3)H]rolipram. [(3)H]piclamilast binding was high-affinity, saturable, reversible, and partially Mg(2+)-dependent. Binding was detected both to membrane and soluble fractions, with K(d) values of 3.1 and 4.5 nM, respectively. The B(max) values for [(3)H]piclamilast were about 1.5-fold greater than that of [(3)H]rolipram binding, suggesting that [(3)H]piclamilast, but not [(3)H]rolipram, binds to LARBS as well as the HARBS. The HARBS was present in all the brain regions examined, but not in peripheral tissues. All PDE4 inhibitors tested were potent competitors for [(3)H]piclamilast binding; the competition curves for rolipram, desmethylpiclamilast, ICI 63,197, and Ro 20-1724 were better described by a two-site model, while the competition curves for piclamilast, cilomilast, roflumilast, and CDP 840 were adequately described by a one-site model. Inhibitors of other PDE families were much less potent. The inhibition of [(3)H]piclamilast was further tested in the presence of 1 microM rolipram to isolate the LARBS. Under this condition, the competition curves for all the inhibitors were adequately described by a one-site model, with K(i) values close to that for the LARBS. The results indicated that [(3)H]piclamilast is a useful tool to directly study inhibitor interaction with the HARBS and the LARBS in rat brain.
Potential antidepressant activity of rolipram and other selective cyclic adenosine 3',5'-monophosphate phosphodiesterase inhibitors.[Pubmed:6302550]
Neuropharmacology. 1983 Mar;22(3):267-72.
Following intraperitoneal administration, the selective cAMP phosphodiesterase (PDE) inhibitors rolipram, ICI 63 197 and Ro 20-1724 were investigated in mice in comparison with imipramine for their effectiveness in two classical test models for prediction of clinical antidepressant activity: antagonism of reserpine-induced hypothermia or hypokinesia and potentiation of yohimbine lethality. Rolipram was approximately 15 times more potent than imipramine or Ro 20-1724 and approximately as potent as ICI 63 197 in antagonizing reserpine-induced hypothermia. The antihypothermic effect of the phosphodiesterase inhibitors occurred at a smaller dose than that of imipramine. In contrast to imipramine, the phosphodiesterase inhibitors reversed reserpine-induced hypokinesia. Rolipram was approximately as potent as ICI 63 197 and about 15 times more potent than Ro 20-1724. Rolipram was approx. 5 times more potent than Ro 20-1724 and approx. as potent as imipramine or ICI 63 197 in potentiating the lethality of yohimbine. In both test models the (-)-isomer of rolipram was approx. 10-15 times more potent than the (+)-isomer, indicating a stereospecific mechanism of action. The present data suggest an antidepressant action of selective cAMP phosphodiesterase inhibitors due to enhancement of central noradrenergic transmission. The hypothesis is put forward that the increase of noradrenaline turnover induced by phosphodiesterase inhibitors in conjunction with the inhibitory action of the compounds on cAMP metabolism enables more efficient adaptative changes to occur at central synapses resulting in a rapid onset of the antidepressant activity. Preliminary results with rolipram in patients with endogenous depression seem to support this assumption.


