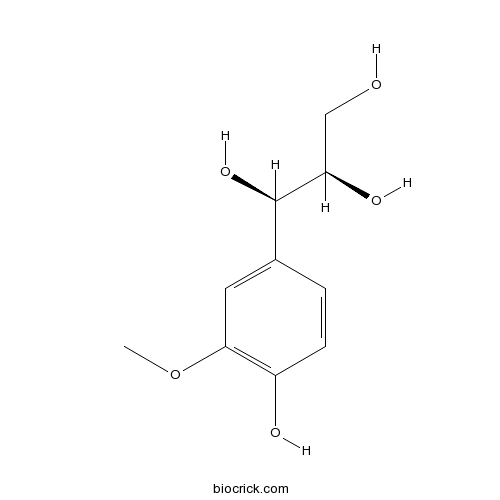
threo-GuaiacylglycerolCAS# 27391-16-8 |
- erythro-Guaiacylglycerol
Catalog No.:BCN5440
CAS No.:38916-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27391-16-8 | SDF | Download SDF |
| PubChem ID | 11063784 | Appearance | Powder |
| Formula | C10H14O5 | M.Wt | 214.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2R)-1-(4-hydroxy-3-methoxyphenyl)propane-1,2,3-triol | ||
| SMILES | COC1=C(C=CC(=C1)C(C(CO)O)O)O | ||
| Standard InChIKey | LSKFUSLVUZISST-PSASIEDQSA-N | ||
| Standard InChI | InChI=1S/C10H14O5/c1-15-9-4-6(2-3-7(9)12)10(14)8(13)5-11/h2-4,8,10-14H,5H2,1H3/t8-,10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

threo-Guaiacylglycerol Dilution Calculator

threo-Guaiacylglycerol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6685 mL | 23.3427 mL | 46.6853 mL | 93.3707 mL | 116.7134 mL |
| 5 mM | 0.9337 mL | 4.6685 mL | 9.3371 mL | 18.6741 mL | 23.3427 mL |
| 10 mM | 0.4669 mL | 2.3343 mL | 4.6685 mL | 9.3371 mL | 11.6713 mL |
| 50 mM | 0.0934 mL | 0.4669 mL | 0.9337 mL | 1.8674 mL | 2.3343 mL |
| 100 mM | 0.0467 mL | 0.2334 mL | 0.4669 mL | 0.9337 mL | 1.1671 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4'-Hydroxy-7-methoxyflavan
Catalog No.:BCN3497
CAS No.:27348-54-5
- VX-765
Catalog No.:BCC3648
CAS No.:273404-37-8
- Precyasterone
Catalog No.:BCN2754
CAS No.:27335-85-9
- 5-Formyl-2-furylboronic acid
Catalog No.:BCC8748
CAS No.:27329-70-0
- Tirapazamine
Catalog No.:BCC5184
CAS No.:27314-97-2
- ICI 63197
Catalog No.:BCC7188
CAS No.:27277-00-5
- Phyllostine
Catalog No.:BCN4773
CAS No.:27270-89-9
- Levobupivacaine HCl
Catalog No.:BCC4675
CAS No.:27262-48-2
- N-(2,6-Dimethylphenyl)-2-piperidinecarboxamide
Catalog No.:BCC9051
CAS No.:27262-40-4
- Neoandrographolide
Catalog No.:BCN4657
CAS No.:27215-14-1
- Cyanidin 3-Arabinoside
Catalog No.:BCC8157
CAS No.:27214-72-8
- Decursidate
Catalog No.:BCN4044
CAS No.:272122-56-2
- Picroside I
Catalog No.:BCN6322
CAS No.:27409-30-9
- Coumarin 7
Catalog No.:BCC8920
CAS No.:27425-55-4
- 5-Methoxyisatin
Catalog No.:BCC8098
CAS No.:39755-95-8
- Xanthohumol D
Catalog No.:BCN5162
CAS No.:274675-25-1
- Neocryptotanshinone II
Catalog No.:BCN3138
CAS No.:27468-20-8
- Ticagrelor
Catalog No.:BCC4975
CAS No.:274693-27-5
- LE 300
Catalog No.:BCC7148
CAS No.:274694-98-3
- 7-Acetoxy-4-methylcoumarin
Catalog No.:BCC8775
CAS No.:2747-05-9
- H-Leu-OtBu.HCl
Catalog No.:BCC2974
CAS No.:2748-02-9
- O-Benzyldauricine
Catalog No.:BCC8222
CAS No.:2748-99-4
- Cyclo(D-Val-L-Pro)
Catalog No.:BCN4015
CAS No.:27483-18-7
- trans-4-Aminocyclohexanol
Catalog No.:BCC9181
CAS No.:27489-62-9
Stereoisomeric guaiacylglycerol-beta-coniferyl aldehyde ether induces distinctive apoptosis by downregulation of MEK/ERK pathway in hepatocellular carcinoma cells.[Pubmed:30196208]
Bioorg Chem. 2018 Dec;81:382-388.
Two 8-O-4'-type neolignan epimers erythro-guaiacylglycerol-beta-coniferyl aldehyde ether (1) and threo-Guaiacylglycerol-beta-coniferyl aldehyde ether (2) were isolated from the stems of Picrasma quassioides. Further chiral separation gave two pairs of enantiomers 1a/1b and 2a/2b. The cytotoxicity assay against hepatocellular carcinoma Hep3B and HepG2 cells was evaluated by MTT assay. The results showed that 1b (IC50=45.56muM) and 2b (IC50=39.02muM) had more cytotoxic effect than its enantiomers 1a (IC50=82.66muM) and 2a (IC50=67.97muM) in Hep3B cells, respectively. Moreover, 1b and 2b could induce more apoptotic cells as well as higher reactive oxygen species (ROS) generation than 1a and 2a at 50muM. In addition, a further study on the phosphoinositide 3-kinase (PI3K)/AKT and mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathways was investigated. The results revealed that all compounds had no significant effect on PI3K/AKT pathway, however, 1b and 2b attenuated the relative levels of p-MEK and p-ERK when compared with 1a and 2a. Taken together, the absolute configurations of guaiacylglycerol-beta-coniferyl aldehyde ether had an impact on the inhibitory effect on Hep3B cells. The inactivation of MEK/ERK signaling pathway might contribute to apoptosis induction and ROS generation in 1b- and 2b-treated cells.
Promotion of mammalian angiogenesis by neolignans derived from soybean extracellular fluids.[Pubmed:29738532]
PLoS One. 2018 May 8;13(5):e0196843.
Excessive or insufficient angiogenesis is associated with major classes of chronic disease. Although less studied, small molecules which can promote angiogenesis are being sought as potential therapeutics for cardiovascular and peripheral arterial disease and stroke. Here we describe a bioassay-directed discovery approach utilising size exclusion and liquid chromatography to purify components of soybean xylem sap that have pro-angiogenic activity. Using high resolution accurate mass spectrometry and nuclear magnetic resonance spectroscopy, the structure of two pro-angiogenic molecules (FK1 and FK2) were identified as erythro-guaiacylglycerol-8-O-4'-(coniferyl alcohol) ether (eGGCE), and threo-Guaiacylglycerol-8-O-4'-(coniferyl alcohol) ether (tGGCE). These two molecules, which are coniferyl neolignan stereoisomers, promoted in vitro angiogenesis in the muM to nM range. Independently sourced samples of eGGCE and tGGCE exhibited comparable pro-angiogenic activity to the soybean derived molecules. The cellular mode of action of these molecules was investigated by studying their effect on endothelial cell proliferation, migration, tube formation and adhesion to the extracellular matrix (ECM) components, fibronectin and vitronectin. They were found to enhance endothelial cell proliferation and endothelial cell tube formation on Matrigel, but did not affect endothelial cell migration or adhesion to fibronectin and vitronectin. Thus, this study has identified two coniferyl neolignan stereoisomers, eGGCE and tGGCE, as pro-angiogenic molecules, with eGGCE being less active than tGGCE.
Chemical constituents from branch of Fraxinus sieboldiana.[Pubmed:26697686]
Zhongguo Zhong Yao Za Zhi. 2015 Jul;40(13):2602-11.
Using a combination of various chromatographic techniques including column chromatography over silica gel, Sephadex LH-20, macroporous adsorbent resin, and reversed-phase HPLC, 115 compounds including diterpenes, sesquiterpenes, treterpenes, coumarins, lignans, fatty acid derivatives, and simple aromatic derivatives were isolated from an ethanol extract of branch of Fraxinus sieboldiana (Oleaceaue), and their structures of the compounds were elucidated by spectroscopic methods including 1 D, 2D NMR and MS techniques. Among them, 41 compounds were new. In previous reports, we have been described the isolation, structure elucidation, and bioactivities of the 41 new compounds and 22 known orii including 8 coumarins, 4 phenolic and 12 phenylethanoidal glycosides. As a consequence, we herein reported the isolation and structure elucidation of the remaining 50 known compounds including 8- hydroxy-12-oxoabieta-9(11),13-dien-20-oic 8, 20-lactone(1), 6beta-hydroxyfcrruginol(2),(+)-pisiferic acid(3), (+)-pisiferal(4),(+)-7-dehydroabiet6none(5), 1-oxomiltirone(6), subdigitatone(7), linarionoside B(8), (9S)-linarionoside B(9), (3R,9R)-3-hydroxy-7,8-dihydro-beta-ionol 9-O-beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranoside(10), ursolic acid(11), betulinic acid(12), euscaphic acid(13), (+)-syringaresinol(14), (+)-fraxiresinol(15), (+)-1-hydroxysyringaresinol(16), pinoresinol(17), medioresinol(18), 8-acetoxypinoresinol(19), epipinoresinol(20), (-)-olivil(21), (+)-cyclo-olivil(22), 3,3'-dimethoxy-4,4',9-trihydroxy-7,9'-epoxylignan-7'-one(23),(+)-1-hydroxypinores inol 4'-O-beta-D-glucopyranoside (24), (+)-1-hydroxypinoresinol 4"-O-beta-D-glucopyranoside(25),(+)-syringaresinol O-beta-D-glucopyranoside (26), liriodendrin (27), ehletianol D(28), icariside E5(29) (-)-(7R, 8R)-threo-1-C-syringylglycerol(30),(-)-(7R, 8S)-erythro-guaiacylglycerol (31),(-)-(7R, 8R)-threo-Guaiacylglycerol(32), 3-(4-beta-D-glucopyranosyloxy-3-methoxy)-phenyl-2E-propenol(33),2,3-dihydroxy-l-( 4-hydroxy-3,5-dimethoxyphenyl)-1-propanone(34), 2,3-dihydroxy-1-(4-hydroxy-3-methoxyphenyl)-1-propanone (35), 3-hydroxy-l-(4-hydroxy-3,5-dimethoxyphenyl)-1-propanone(36), omega-hydroxypropioguaiacone(37), sinapyladehyde(38), trans-p-hydroxycinnamaldehyde(39), syringic acid(40), vanilic acid(41), vanillin(42), 4-hydroxy-benzaldehyde (43), (24R)-24-ethyl-5alpha-cholestane-3beta,5,6beta-triol(44), beta-sitosterol(45), daucosterol(46), 2,6-dimethoxy-I,4-benzoquinone(47), 2,6-dimethoxy-pyran-4-one(48), 1-(beta-D-ribofuranosyl)uracil(49), and mannitol(50). Compouds 1-7,12,18,28-37,44 and 48 were obtained from the genus Fraxinus for the first time.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica var. formosana cv. Chuanbaizhi].[Pubmed:26552172]
Zhongguo Zhong Yao Za Zhi. 2015 Jun;40(11):2148-56.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica var. formosana cv. Chuanbaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Twenty-nine compounds were obtained and identified as isoimperatorin (1), beta-sitosterol (2), imperatorin (3), bergapten (4), osthenol (5), xanthotoxin (6), isoimpinellin (7), dehydrogeijerin (8), phellopterin (9), isodemethylfuropinarine (10), 7-demethylsuberosin (11), alloimperatorin (12), xanthotoxol (13), isooxypeucedanin (14), alloisoimperatorin (15), demethylfuropinarine (16), 5-hydroxy-8-methoxypsoralen (17), oxypeucedanin methanolate (18), pabulenol (19), byakangelicin (20), marmesin (21), (+) -decursinol (22), heraclenol (23), oxypeucedanin hydrate (24), marmesinin (25), ulopterol (26), erythro-guaiacylglycerol-beta-ferulic acid ether (27), threo-Guaiacylglycerol-beta-ferulic acid ether (28), and uracil (29). Compounds 5, 8, 11, 18, 21-23, and 26-28 were obtained from the roots of title plant for the first time.
[Lignans isolated from stems of Sambucus williamsii and their proliferation effects on UMR106 cells].[Pubmed:25272496]
Zhongguo Zhong Yao Za Zhi. 2014 Jul;39(14):2684-8.
The present study aims to investigate the lignan constituents from Sambucus williamsii and their proliferation effects on osteoblast-like UMR106 cells. Seven compounds were isolated and purified by macroporous resin D101, silica gel, Sephadex LH-20, Toyopearl HW-40, ODS column chromatographies and Preparative HPLC(C-18). Their structures were elucidated by spectroscopic methods as threo-Guaiacylglycerol-beta-0-4'-conifery ether (1), lirioresinol A (2), 1-hydroxypinoresinol (3), 5-methoxybalanophonin (4), balanophonin (5), 5-methoxy-trans-dihydrodehydrodiconiferyl alcohol (6), and p-hydroxybenzaldehyde (7). Compounds 3-7 were obtained from this genus for the first time. The proliferation effects of all isolated compounds on osteoblast-like UMR106 cells were determined. Compounds 1-7 (1 x 10(-12)-1 x 10(-7) mol x L(-1)) increased UMR106 cell proliferation to some extent.
Lignans from the stems of Clematis armandii ("Chuan-Mu-Tong") and their anti-neuroinflammatory activities.[Pubmed:24661966]
J Ethnopharmacol. 2014 May 14;153(3):737-43.
ETHNOPHARMACOLOGICAL RELEVANCE: The dried stems of Clematis armandii (Caulis clematidis armandii), named "Chuan-Mu-Tong" in Chinese Pharmacopoeia, have been traditionally used as an herbal remedy mainly for inflammation-associated diseases. The Aim of the study is to identify the potential anti-neuroinflammatory components from Clematis armandii. MATERIALS AND METHODS: The ethanol extract of "Chuan-Mu-Tong" was suspended in H(2)O and exhaustively extracted with CH(2)Cl(2). The CH(2)Cl(2) fraction was successively subjected to column chromatography (CC) over silica gel, Sephadex LH-20, and semi-preparative HPLC. The structures of the isolated compounds were identified by spectroscopic methods and by comparison with those reported in the literature. Their anti-neuroinflammatory activities were evaluated by inhibitory effects on pro-inflammatory mediators [e.g. nitric oxide (NO) and tumor necrosis factor-alpha (TNF-alpha)] in lipopolysaccharide (LPS)-activated BV-2 cells. RESULTS: One new and sixteen known lignans were isolated and characterized. The absolute configuration of the new lignan, (7R,8S)-9-acetyl-dehydrodiconiferyl alcohol (1), was elucidated by a combination of 1D/2D NMR techniques and the Electronic Circular Dichroism (ECD) spectroscopy based on the empirical helicity rules. The anti-neuroinflammatory bioassay showed that compounds 1, (7R,8S)-dehydrodiconiferyl alcohol (2), erythro-guaiacylglycerol-beta-coniferyl ether (5), and threo-Guaiacylglycerol-beta-coniferyl ether (6) displayed significant inhibitory effects on NO production. Among them, neolignans 1 and 2 exhibited more potent activities than the positive control (N(G)-monomethyl-L-arginine, L-NMMA), with an IC(5)(0) value of 9.3 and 3.9 muM, respectively. Moreover, both 1 and 2 were also found to concentration-dependently suppress the TNF-alpha release in LPS-stimulated BV-2 cells. CONCLUSION: The results revealed that lignans are the major components of "Chuan-Mu-Tong", and their anti-neuroinflammatory activities strongly support the traditional application of this herb medicine on inflammation. Moreover, the dihydrobenzo[b]furan neolignans 1 and 2 as well as Caulis clematidis armandii could be further exploited as new therapeutic agents to treat inflammation-mediated neurodegenerative and aging-associated diseases.
In vitro activity-guided identification of antioxidants in aged garlic extract.[Pubmed:23448127]
J Agric Food Chem. 2013 Mar 27;61(12):3059-67.
Activity-guided fractionation was applied on an aged garlic extract (AGE), reported to show strong antioxidant activity, in order to locate the key in vitro antioxidant ingredients by means of the hydrogen peroxide scavenging (HPS) assay as well as the ORAC assay. Besides the previously reported four tetrahydro-beta-carbolines, (1R,3S)- and (1S,3S)-1-methyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid and (1R,3S)- and (1S,3S)-1-methyl-1,2,3,4-tetrahydro-beta-carboline-1,3-dicarboxylic acid, LC-MS/MS, LC-TOF-MS, and 1D/2D-NMR experiments led to the identification of coniferyl alcohol and its dilignols (-)-(2R,3S)-dihydrodehydrodiconiferyl alcohol, (+)-(2S,3R)-dehydrodiconiferyl alcohol, erythro-guaiacylglycerol-beta-O-4'-coniferyl ether, and threo-Guaiacylglycerol-beta-O-4'-coniferyl ether as the major antioxidants in AGE. The purified individual compounds showed high antioxidant activity, with EC50 values of 9.7-11.8 muM (HPS assay) and 2.60-3.65 mumol TE/mumol (ORAC assay), respectively.
[Lignans from Sinocalamus affinis].[Pubmed:23019880]
Zhongguo Zhong Yao Za Zhi. 2012 Jul;37(13):1968-72.
Fifteen compounds were isolated from the stem (with skin removed) of Sinocalamus affinis by a combination of various chromatographic techniques including silica gel, macroporous adsorbent resin, Sephadex LH-20, and preparative HPLC. Their structures were elucidated by spectroscopic data as ( + )-(1S, 2R)-1, 2-bis (4-hydroxy- 3-methoxyphenyl)-1, 3-propanediol (1), threo-Guaiacylglycerol-beta-O-4'-coniferyl ether(2), erythro-guaiacylglycerol-beta-O-4'-coniferyl ether(3), ( + )-(7S, 8R, 8'R)-5'-methoxylariciresinol(4), ( + )-(7S, 8R, 8'R)-5, 5'-dimethoxylariciresinol (5), ( +/- )-glaberide I (6), ( - )-syringaresinol (7), ( - )-medioresinol(8), ( - )-(8R, 8R')-4, 4'-dihydroxy-3, 3', 5, 5'-tetramethoxyligna-9, 9'-diol(9), ( - )-secoisolariciresinol-9, 9'-acetonide (10), and ( + )-lyoniresinol (11); a new natural product 2, 6-dimethoxypyran4-one (12), and beta-sitosterol, 4-hydroxycinnamic acid, and 2, 6-dimethoxy-p-benzoquinone. These compounds were isolated from the genus Sinocalamus for the first time, compound 10 should be an artifact.
[Non-alkaloid chemical constituents from Coptis chinensis].[Pubmed:22803368]
Zhongguo Zhong Yao Za Zhi. 2012 May;37(9):1241-4.
OBJECTIVE: To separate and identify chemical constituents from Coptis chinensis. METHOD: The compounds were separated and purified by various chromatographic techniques. Their structures were identified on the basis of their physicochemical properties using spectral techniques such as NMR and MS. RESULT: Thirteen compounds were separated from ethanol extracts of C. chinensis, including seven lignans, three simple phenylpropanoids, two flavones and one phenolic acid, and identified as erythro-guaiacylglycerol-8-O-4'-(coniferyl alcohol) ether (1), threo-Guaiacylglycerol-8-O-4'-(coniferyl alcohol) ether (2), (+)-pinoresinol (3), (+)-medioresinol (4), (+)-lariciresinol (5), (+)-5'-methoxylariciresinol (6), (+)-isolariciresinol (7), chlorogenic acid (8), ferulic acid (9), Z-octadecyl caffeate (10), rhamnetin (11), wogonin (12), and vanillic acid (13). CONCLUSION: Compounds 1, 2, 4, 6, 10-13 were separated from the genus Coptis for the first time.
Further investigation into maple syrup yields 3 new lignans, a new phenylpropanoid, and 26 other phytochemicals.[Pubmed:21675726]
J Agric Food Chem. 2011 Jul 27;59(14):7708-16.
Maple syrup is made by boiling the sap collected from certain maple ( Acer ) species. During this process, phytochemicals naturally present in tree sap are concentrated in maple syrup. Twenty-three phytochemicals from a butanol extract of Canadian maple syrup (MS-BuOH) had previously been reported; this paper reports the isolation and identification of 30 additional compounds (1-30) from its ethyl acetate extract (MS-EtOAc) not previously reported from MS-BuOH. Of these, 4 compounds are new (1-3, 18) and 20 compounds (4-7, 10-12, 14-17, 19, 20, 22-24, 26, and 28-30) are being reported from maple syrup for the first time. The new compounds include 3 lignans and 1 phenylpropanoid: 5-(3'',4''-dimethoxyphenyl)-3-hydroxy-3-(4'-hydroxy-3'-methoxybenzyl)-4-(hydroxym ethyl)dihydrofuran-2-one (1), (erythro,erythro)-1-[4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl) ethoxy]-3,5-dimethoxyphenyl]-1,2,3-propanetriol (2), (erythro,threo)-1-[4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)et hoxy]-3,5-dimethoxyphenyl]-1,2,3-propanetriol (3), and 2,3-dihydroxy-1-(3,4- dihydroxyphenyl)-1-propanone (18), respectively. In addition, 25 other phenolic compounds were isolated including (threo,erythro)-1-[4-[(2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)e thoxy]-3-methoxyphenyl]-1,2,3-propanetriol (4), (threo,threo)-1-[4-[(2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)eth oxy]-3-methoxyphenyl]-1,2,3-propanetriol (5), threo-Guaiacylglycerol-beta-O-4'-dihydroconiferyl alcohol (6), erythro-1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxypropyl)-2,6-dimethoxyphenoxy ]-1,3-propanediol (7), 2-[4-[2,3-dihydro-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2-benzofuranyl] -2,6-dimethoxyphenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol (8), acernikol (9), leptolepisol D (10), buddlenol E (11), (1S,2R)-2-[2,6-dimethoxy-4-[(1S,3aR,4S,6aR)-tetrahydro-4-(4-hydroxy-3,5-dimethoxy phenyl)-1H,3H-furo[3,4-c]furan-1-yl]phenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-pr opanediol (12), syringaresinol (13), isolariciresinol (14), icariside E4 (15), sakuraresinol (16), 1,2-diguaiacyl-1,3-propanediol (17), 2,3-dihydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-1-propanone (19), 3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one (20), dihydroconiferyl alcohol (21), 4-acetylcatechol (22), 3',4',5'-trihydroxyacetophenone (23), 3,4-dihydroxy-2-methylbenzaldehyde (24), protocatechuic acid (25), 4-(dimethoxymethyl)pyrocatechol (26), tyrosol (27), isofraxidin (28), and 4-hydroxycatechol (29). One sesquiterpene, phaseic acid (30), which is a known metabolite of the phytohormone abscisic acid, was also isolated from MS-EtOAc. The antioxidant activities of MS-EtOAc (IC(50) = 75.5 mug/mL) and the pure isolates (IC(50) ca. 68-3000 muM) were comparable to that of vitamin C (IC(50) = 40 muM) and the synthetic commercial antioxidant butylated hydroxytoluene (IC(50) = 3000 muM), in the diphenylpicrylhydrazyl radical scavenging assay. The current study advances scientific knowledge of maple syrup constituents and suggests that these diverse phytochemicals may impart potential health benefits to this natural sweetener.
Gentisic acid conjugates of Medicago truncatula roots.[Pubmed:19683320]
Phytochemistry. 2009 Jul;70(10):1272-6.
Three phenolic glycosides 5-O-{[5''-O-E-(4'''-O-threo-Guaiacylglycerol)-feruloyl]-beta-apiofuranosyl-(1-->2 )-beta-xylopyranosyl} gentisic acid, 5-O-[(5''-O-vanilloyl)-beta-apiofuranosyl-(1-->2)-beta-xylopyranosyl] gentisic acid and 1-O-[E-(4'''-O-threo-Guaiacylglycerol)-feruloyl]-3-O-beta-galacturonopyranosyl glycerol were isolated and identified from the roots of Medicago truncatula together with four known 5-O-beta-xylopyranosyl gentisic acid, vicenin-2, hovetrichoside C and pterosupin identified for the first time in this species. Structural elucidation was carried out on the basis of UV, mass, (1)H and (13)C NMR spectral data.
[Isolation and identification of compounds from marine mangrove plant Avicennia marina].[Pubmed:19377635]
Beijing Da Xue Xue Bao Yi Xue Ban. 2009 Apr 18;41(2):221-5.
OBJECTIVE: To investigate the chemical constituents from Avicennia marina. METHODS: The isolation and purification of the CH2Cl2 and n-BuOH fractions of this plant were performed, and the chemical structures were elucidated by spectral analysis as well as comparison of their spectral data with literature values. RESULTS: Three novel compounds were obtained and identified as erythro-guaiacylglycerol-beta-ferulic acid ether (1), marinnone A (16) and marinnone B (17), along with eighteen known compounds as threo-Guaiacylglycerol-beta-ferulic acid ether (2), eleutheroside E2 (3), (+)-lirioresinol A (4), dihydroxymethyl-bis (3, 5-dimethoxy-4-hydroxyphenyl) tetrahydrofuran-9-O-beta-glucopyranoside (5), (+)-lyoniresinol 3a-O-beta-D-glucopyranoside (6), (-)-lyoniresinol 3a-O-beta-D-glucopyranoside (7), epi-pinoresinol (8), leucoseceptoside A (9), jionoside C (10), salsaside A (11), ilicifolioside A (12), acteoside (13), isoacteoside (14), ethyl ferulate (15), avicennone D (18), avicenone E (19), avicennol C (20), and stenocarpoquinone B (21). CONCLUSION: Three new compounds (1, 16 and 17) were obtained and thirteen known compounds, 2-12, 14 and 15 were isolated from Avicennia genus for the first time.
Two new neolignan glycosides from leaves of Osmanthus heterophyllus.[Pubmed:19184274]
J Nat Med. 2009 Apr;63(2):227-31.
Two new neolignan glycosides, (7R, 8R)-threo-Guaiacylglycerol-8-O-4'-sinapyl ether 7-O-beta-D: -glucopyranoside (1) and (7S, 8R)-5-methoxydehydrodiconiferyl alcohol 4-O-beta-D: -glucopyranoside (2), and four known ones (3-6), were isolated from the leaves of Osmanthus heterophyllus. The structures of compounds 1-6 were established on the basis of spectral and chemical data.
Lignans isolated from Campylotropis hirtella (Franch.) Schindl. decreased prostate specific antigen and androgen receptor expression in LNCaP cells.[Pubmed:18656936]
J Agric Food Chem. 2008 Aug 27;56(16):6928-35.
Accumulating epidemiological data suggest that Asian men have lower incidences of prostate cancer and benign prostate hyperplasia (BPH) compared with American and European populations and may have benefited from their higher intake of phytoestrogens in their diet. However, how these phytochemicals affect prostatic diseases is still unclear. In this study, we isolated six lignans from a plant, Campylotropis hirtella (Franch.) Schindl., which has been used as a folk medicine for treatment of BPH in China, through bioassay guided fractionation. They were dehydrodiconiferyl alcohol (C1), 4-[(-6-hydroxy-2,3-dihydro-1-benzofuran-3-yl)methyl]-5-methoxybenzene-1,3-diol (C2), erythro-guaiacylglycerol-beta-O-4'-coniferyl ether (C3), threo-Guaiacylglycerol-beta-O-4'-coniferyl ether (C4), secoisolariciresinol (C5), and prupaside (C6), where C2 was identified as a new lignan analog. Their IC50 values for inhibition of prostate specific antigen (PSA) secretion were 19, 45, 110, 128, 137, and 186 microM, respectively, from C1 to C6 in LNCaP cells. Further study showed that C1-5 down-regulated cellular PSA expression and C1-4 also decreased androgen receptor (AR) expression in LNCaP cells. Furthermore, we investigated the proapoptotic effect of C1 on LNCaP cells. The active forms of caspase 3 associated with the specific proteolysis of poly (ADP-ribose) polymerase (PARP) were detected, and the antiapoptotic protein Bcl-2 was down-regulated after the treatment with C1. These results collectively indicated that these lignans may have chemopreventive or therapeutic actions for prostate cancer through suppressing AR signaling pathway and inducing apoptosis.
Antioxidative compounds from Quercus salicina Blume stem.[Pubmed:18409038]
Arch Pharm Res. 2008 Mar;31(3):274-8.
The chromatographic separation of MeOH extract from the Quercus salicina Blume Stem led to the isolation of five phenolic compounds. Using spectroscopic methods, the structures of these compounds were determined as D-threo-Guaiacylglycerol 8-O-beta-D-(6'-O-galloyl)glucopyranoside (1), 9-methoxy-D-threo-Guaiacylglycerol 8-O-beta-D-(6'-O-galloyl)glucopyranoside (2), 6''-O-galloyl salidroside (3), methyl gallate (4), quercetin (5). We measured radical scavenging activity with the DPPH method and the anti-lipid peroxidative efficacy on human LDL with TBARS assay, with the result that all these compounds exhibited the antioxidative activity.


