TirapazamineAnticancer drug CAS# 27314-97-2 |
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- TPT-260 Dihydrochloride
Catalog No.:BCC5172
CAS No.:2076-91-7
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27314-97-2 | SDF | Download SDF |
| PubChem ID | 33776 | Appearance | Powder |
| Formula | C7H6N4O2 | M.Wt | 178.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SR259075; SR4233; Win59075 | ||
| Solubility | DMSO : ≥ 39 mg/mL (218.92 mM) *"≥" means soluble, but saturation unknown. | ||
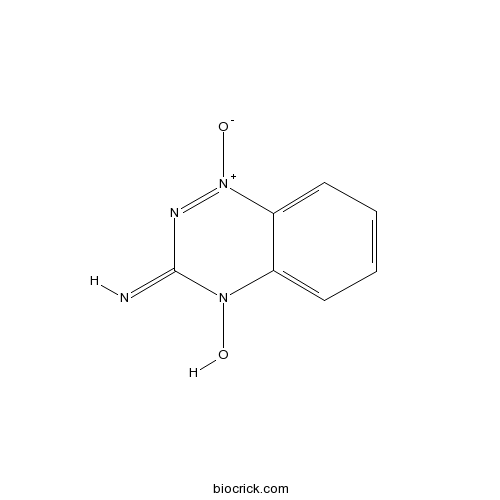
| Chemical Name | 4-hydroxy-1-oxido-1,2,4-benzotriazin-1-ium-3-imine | ||
| SMILES | C1=CC=C2C(=C1)N(C(=N)N=[N+]2[O-])O | ||
| Standard InChIKey | QVMPZNRFXAKISM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H6N4O2/c8-7-9-11(13)6-4-2-1-3-5(6)10(7)12/h1-4,8,12H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tirapazamine(SR259075; Win59075; SR4233) is an experimental anticancer drug that is activated to a toxic radical only at very low levels of oxygen; a phenomenon known as tumor hypoxia.
IC50 value:
Target:
in vitro: Tirapazamine could downregulate HIF-1α expression by decreasing HIF-1α protein synthesis. The enhanced apoptosis induced by tirapazamine plus SN-38 (the active metabolite of irinotecan) was accompanied by increased mitochondrial depolarization and caspase pathway activation [1].
in vivo: The combination treatment dramatically inhibited the accumulation of HIF-1α protein, decreased the HIF-1α transcriptional activation, and impaired the phosphorylation of proteins involved in the homologous recombination repair pathway, ultimately resulting in the synergism of these two drugs. Moreover, the increased anticancer efficacy of tirapazamine combined with irinotecan was further validated in a human liver cancer Bel-7402 xenograft mouse model [1]. Rats were intraperitoneally injected six times once a week with tirapazamine in two doses, 5 (5TP) and 10 mg/kg (10TP), while doxorubicin was administered in dose 1.8 mg/kg (DOX). Subsequent two groups received both drugs simultaneously (5TP+DOX and 10TP+DOX). Tirapazamine reduced heart lipid peroxidation and normalised RyR2 protein level altered by doxorubicin [2]. References: | |||||

Tirapazamine Dilution Calculator

Tirapazamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6132 mL | 28.0662 mL | 56.1325 mL | 112.2649 mL | 140.3312 mL |
| 5 mM | 1.1226 mL | 5.6132 mL | 11.2265 mL | 22.453 mL | 28.0662 mL |
| 10 mM | 0.5613 mL | 2.8066 mL | 5.6132 mL | 11.2265 mL | 14.0331 mL |
| 50 mM | 0.1123 mL | 0.5613 mL | 1.1226 mL | 2.2453 mL | 2.8066 mL |
| 100 mM | 0.0561 mL | 0.2807 mL | 0.5613 mL | 1.1226 mL | 1.4033 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
Tirapazamine (SR259075; Win59075; SR4233) is an experimental anticancer drug that is activated to a toxic radical only at very low levels of oxygen; a phenomenon known as tumor hypoxia.
In vitro: Tirapazamine could downregulate HIF-1α expression by reducing HIF-1α protein synthesis. The enhanced apoptosis induced by tirapazamine plus SN-38 (the active metabolite of irinotecan) was followed by increased mitochondrial depolarization and caspase pathway activation [1].
In vivo: Tirapazamine plus SN-38 treatment dramatically blocked the accumulation of HIF-1α protein, reduced the HIF-1α transcriptional activation, and weakened the phosphorylation of proteins in the homologous recombination repair pathway, ultimately resulting in the synergism of these two drugs. Moreover, the increased anticancer efficacy of tirapazamine combined with irinotecan played a further role on a human liver cancer Bel-7402 xenograft mouse model [1]. Rats were intraperitoneally injected six times once a week with tirapazamine in two doses [5 (5TP) and 10 mg (10TP)], while doxorubicin was administered in dose 1.8 mg (DOX). Subsequent two groups received both drugs at the same time (5TP+DOX and 10TP+DOX). Tirapazamine decreased heart lipid peroxidation and RyR2 protein was up to normal level altered by doxorubicin [2].
Clinical trial: Clinical study has been conducted.
References:
[1]. Cai TY1, Liu XW, Zhu H, Cao J, Zhang J, Ding L, Lou JS, He QJ, Yang B. Tirapazamine sensitizes hepatocellular carcinoma cells to topoisomerase I inhibitors via cooperative modulation of hypoxia-inducible factor-1α. Mol Cancer Ther. 2014 Mar;13(3):630-42.
[2]. Sliwinska J1, Dudka J, Korga A, Burdan F, Matysiak W, Jodlowska-Jedrych B, Mandziuk S, Dawidek-Pietryka K.Tirapazamine-doxorubicin interaction referring to heart oxidative stress and Ca balance protein levels. Oxid Med Cell Longev. 2012;2012:890826.
- ICI 63197
Catalog No.:BCC7188
CAS No.:27277-00-5
- Phyllostine
Catalog No.:BCN4773
CAS No.:27270-89-9
- Levobupivacaine HCl
Catalog No.:BCC4675
CAS No.:27262-48-2
- N-(2,6-Dimethylphenyl)-2-piperidinecarboxamide
Catalog No.:BCC9051
CAS No.:27262-40-4
- Neoandrographolide
Catalog No.:BCN4657
CAS No.:27215-14-1
- Cyanidin 3-Arabinoside
Catalog No.:BCC8157
CAS No.:27214-72-8
- Decursidate
Catalog No.:BCN4044
CAS No.:272122-56-2
- Pedunsaponin C
Catalog No.:BCN8193
CAS No.:272120-53-3
- Miltirone
Catalog No.:BCN5356
CAS No.:27210-57-7
- Polydatin
Catalog No.:BCN5949
CAS No.:27208-80-6
- Bis[4-(2-hydroxyethoxy)phenyl] sulfone
Catalog No.:BCC8888
CAS No.:27205-03-4
- Ampelopsin
Catalog No.:BCN5160
CAS No.:27200-12-0
- 5-Formyl-2-furylboronic acid
Catalog No.:BCC8748
CAS No.:27329-70-0
- Precyasterone
Catalog No.:BCN2754
CAS No.:27335-85-9
- VX-765
Catalog No.:BCC3648
CAS No.:273404-37-8
- 4'-Hydroxy-7-methoxyflavan
Catalog No.:BCN3497
CAS No.:27348-54-5
- threo-Guaiacylglycerol
Catalog No.:BCN5161
CAS No.:27391-16-8
- Picroside I
Catalog No.:BCN6322
CAS No.:27409-30-9
- Coumarin 7
Catalog No.:BCC8920
CAS No.:27425-55-4
- 5-Methoxyisatin
Catalog No.:BCC8098
CAS No.:39755-95-8
- Xanthohumol D
Catalog No.:BCN5162
CAS No.:274675-25-1
- Neocryptotanshinone II
Catalog No.:BCN3138
CAS No.:27468-20-8
- Ticagrelor
Catalog No.:BCC4975
CAS No.:274693-27-5
- LE 300
Catalog No.:BCC7148
CAS No.:274694-98-3
The synergistic radiosensitizing effect of tirapazamine-conjugated gold nanoparticles on human hepatoma HepG2 cells under X-ray irradiation.[Pubmed:27555772]
Int J Nanomedicine. 2016 Jul 28;11:3517-31.
Reductive drug-functionalized gold nanoparticles (AuNPs) have been proposed to enhance the damage of X-rays to cells through improving hydroxyl radical production by secondary electrons. In this work, polyethylene glycol-capped AuNPs were conjugated with Tirapazamine (TPZ) moiety, and then thioctyl TPZ (TPZs)-modified AuNPs (TPZs-AuNPs) were synthesized. The TPZs-AuNPs were characterized by transmission electron microscopy, ultraviolet-visible spectra, dynamic light scattering, and inductively coupled plasma mass spectrometry to have a size of 16.6+/-2.1 nm in diameter and a TPZs/AuNPs ratio of ~700:1. In contrast with PEGylated AuNPs, the as-synthesized TPZs-AuNPs exhibited 20% increment in hydroxyl radical production in water at 2.0 Gy, and 19% increase in sensitizer enhancement ratio at 10% survival fraction for human hepatoma HepG2 cells under X-ray irradiation. The production of reactive oxygen species in HepG2 cells exposed to X-rays in vitro demonstrated a synergistic radiosensitizing effect of AuNPs and TPZ moiety. Thus, the reductive drug-conjugated TPZs-AuNPs as a kind of AuNP radiosensitizer with low gold loading provide a new strategy for enhancing the efficacy of radiation therapy.
Cytotoxicity of Tirapazamine (3-Amino-1,2,4-benzotriazine-1,4-dioxide)-Induced DNA Damage in Chicken DT40 Cells.[Pubmed:27943678]
Chem Res Toxicol. 2017 Feb 20;30(2):699-704.
Tirapazamine (TPZ) is an anticancer drug with highly selective cytotoxicity toward hypoxic cells. TPZ is converted to a radical intermediate under hypoxic conditions, and this intermediate interacts with intracellular macromolecules, including DNA. TPZ has been reported to indirectly induce DNA double-strand breaks (DSBs) through the formation of various intermediate DNA lesions under hypoxic conditions. Although the topoisomerase II-DNA complex has been identified as one of these intermediates, other lesions have not yet been defined. In order to obtain a deeper understanding of the mechanisms responsible for the selective cytotoxicity of TPZ toward hypoxic cells, its cellular sensitivity was systematically examined with genetically isogenic DNA-repair-deficient mutant DT40 cell lines. Our results showed that tdp1(-/-), tdp2(-/-), parp1(-/-), and aptx1(-/-) cells displayed hypersensitivity to TPZ only under hypoxic conditions. These results strongly suggest that the accumulation of the topoisomerase I-trapped DNA complex, topoisomerase II-trapped DNA complex, and abortive ligation products with 5'-AMP are the potential causes of TPZ-induced hypoxic cell death. Furthermore, our genetic analysis revealed that under normoxic conditions (as well as hypoxic conditions), TPZ exhibited significant cytotoxicity toward cell lines deficient in homologous recombination, nonhomologous end joining, base excision repair, and translesion synthesis. Ascorbic acid, a radical scavenger, suppressed TPZ-induced cytotoxicity toward normoxic cells. These results suggest the involvement of oxidative DNA damage and DSBs produced by reactive oxygen species generated from superoxide, a byproduct of the oxidation of TPZ radical intermediates in normoxic cells. Collectively, our results demonstrate that TPZ induces oxidative DNA damage under normoxic and hypoxic conditions and selectively introduces abortive topoisomerase-DNA complexes and unligatable DNA ends under hypoxic conditions.
Tirapazamine has no Effect on Hepatotoxicity of Cisplatin and 5-fluorouracil but Interacts with Doxorubicin Leading to Side Changes in Redox Equilibrium.[Pubmed:26990033]
Basic Clin Pharmacol Toxicol. 2016 Sep;119(3):330-40.
Tirapazamine is a hypoxia-activated prodrug which was shown to exhibit up to 300 times greater cytotoxicity under anoxic in comparison with aerobic conditions. Thus, the combined anticancer therapy of Tirapazamine with a routinely used anticancer drug seems to be a promising solution. Because Tirapazamine undergoes redox cycle transformation in this study, the effect of Tirapazamine on redox hepatic equilibrium, lipid status and liver morphology was evaluated in rats exposed to cisplatin, doxorubicin and 5-fluorouracil. Rats were intraperitoneally injected with Tirapazamine and a particular cytostatic. The animals were killed, and blood and liver were collected. Hepatic glucose, total cholesterol, triglycerides, NADH, NADPH glutathione and the activity of glucose-6-phosphate dehydrogenase were determined. Liver morphology and the immune expression of HMG-CoA-reductase were also assessed. Glucose, total cholesterol, triglycerides, bilirubin concentrations and the activity of aspartate and alanine aminotransferases were determined in the plasma. Tirapazamine displayed insignificant interactions with cisplatin and 5-fluorouracil referring to hepatic morphology and biochemical parameters. However, Tirapazamine interacts with doxorubicin, thus leading to side changes in redox equilibrium and lipid peroxidation, but those effects are not severe enough to exclude that drug combination from further studies. Thus, Tirapazamine seems to be a promising agent in successive studies on anticancer activity in similar schedules.
Hypoxia-activated cytotoxic agent tirapazamine enhances hepatic artery ligation-induced killing of liver tumor in HBx transgenic mice.[Pubmed:27702890]
Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):11937-11942.
Transarterial chemoembolization (TACE) is the main treatment for intermediate stage hepatocellular carcinoma (HCC) with Barcelona Clinic Liver Cancer classification because of its exclusive arterial blood supply. Although TACE achieves substantial necrosis of the tumor, complete tumor necrosis is uncommon, and the residual tumor generally rapidly recurs. We combined Tirapazamine (TPZ), a hypoxia-activated cytotoxic agent, with hepatic artery ligation (HAL), which recapitulates transarterial embolization in mouse models, to enhance the efficacy of TACE. The effectiveness of this combination treatment was examined in HCC that spontaneously developed in hepatitis B virus X protein (HBx) transgenic mice. We proved that the tumor blood flow in this model was exclusively supplied by the hepatic artery, in contrast to conventional orthotopic HCC xenografts that receive both arterial and venous blood supplies. At levels below the threshold oxygen levels created by HAL, TPZ was activated and killed the hypoxic cells, but spared the normoxic cells. This combination treatment clearly limited the toxicity of TPZ to HCC, which caused the rapid and near-complete necrosis of HCC. In conclusion, the combination of TPZ and HAL showed a synergistic tumor killing activity that was specific for HCC in HBx transgenic mice. This preclinical study forms the basis for the ongoing clinical program for the TPZ-TACE regimen in HCC treatment.


