LatinoneCAS# 79157-36-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 79157-36-1 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Red powder |
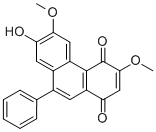
| Formula | C22H16O5 | M.Wt | 360.4 |
| Type of Compound | Other Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Latinone Dilution Calculator

Latinone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7747 mL | 13.8735 mL | 27.7469 mL | 55.4939 mL | 69.3674 mL |
| 5 mM | 0.5549 mL | 2.7747 mL | 5.5494 mL | 11.0988 mL | 13.8735 mL |
| 10 mM | 0.2775 mL | 1.3873 mL | 2.7747 mL | 5.5494 mL | 6.9367 mL |
| 50 mM | 0.0555 mL | 0.2775 mL | 0.5549 mL | 1.1099 mL | 1.3873 mL |
| 100 mM | 0.0277 mL | 0.1387 mL | 0.2775 mL | 0.5549 mL | 0.6937 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Juncutol
Catalog No.:BCN9292
CAS No.:1021950-14-0
- Lycojaponicuminol C
Catalog No.:BCN9291
CAS No.:1651839-34-7
- 6'-O-Galloylsalidroside
Catalog No.:BCN9290
CAS No.:83013-86-9
- Rubanthrone A
Catalog No.:BCN9289
CAS No.:441764-20-1
- Browniine
Catalog No.:BCN9288
CAS No.:5140-42-1
- Oregonin
Catalog No.:BCN9287
CAS No.:55303-93-0
- Palmitone
Catalog No.:BCN9286
CAS No.:502-73-8
- Phoyunbene C
Catalog No.:BCN9285
CAS No.:886747-63-3
- 1-O-p-Coumaroylglycerol
Catalog No.:BCN9284
CAS No.:106055-11-2
- N1,N5,N10-Tri-p-coumaroylspermidine
Catalog No.:BCN9283
CAS No.:131086-78-7
- Juncuenin D
Catalog No.:BCN9282
CAS No.:1161681-24-8
- 9,10-Didehydroeffususol A
Catalog No.:BCN9281
CAS No.:1869082-57-4
- Dehydrojuncuenin B
Catalog No.:BCN9294
CAS No.:1161681-28-2
- Isoboldine
Catalog No.:BCN9295
CAS No.:3019-51-0
- (+)-Maackiain
Catalog No.:BCN9296
CAS No.:23513-53-3
- 16-Oxolycoclavanol
Catalog No.:BCN9297
CAS No.:53800-21-8
- Changweikang aldehyde
Catalog No.:BCN9298
CAS No.:2119605-13-7
- Ugandenial A
Catalog No.:BCN9299
CAS No.:1175880-15-5
- Pholidotol C
Catalog No.:BCN9300
CAS No.:1013909-91-5
- 1,6-Dimethyl-4,5-dihydropyrene-2,7-diol
Catalog No.:BCN9301
CAS No.:2255331-28-1
- ent-13,16β,17-Trihydroxykauran-19-oic acid
Catalog No.:BCN9302
CAS No.:142543-30-4
- n-Butyl β-D-fructofuranoside
Catalog No.:BCN9303
CAS No.:80971-60-4
- Taxilluside A
Catalog No.:BCN9304
CAS No.:1661914-46-0
- 3,5,3'-Trihydroxybibenzyl
Catalog No.:BCN9305
CAS No.:86630-23-1
Neoflavonoids as potential osteogenic agents from Dalbergia sissoo heartwood.[Pubmed:24803361]
Bioorg Med Chem Lett. 2014 Jun 15;24(12):2664-8.
The present study was undertaken to investigate and rationalize the in vitro antiosteoporotic activity of neoflavonoids, isolated from Dalbergia sissoo heartwood. Neoflavonoids were isolated using extensive column chromatography and identified as dalsissooal (1) a new compound and cearoin (2), dalbergin (3), 4-methoxy dalbergion (4), dalbergiphenol (5), dalbergichromene (6), methyl dalbergin (7) and Latinone (8) as known compounds by comparison their spectroscopic data with those reported in the literature. Among the screened compounds, compounds 1, 3, 5-8 significantly increased proliferation as assessed by alkaline phosphatase activity and mineralization in calvarial osteoblast cells.
Structure-activity relationships in allergic contact dermatitis. Part III. The sensitizing capacity of substituted phenanthrenequinones: a quantum-mechanical approach.[Pubmed:14749026]
Am J Contact Dermat. 2003 Jun;14(2):82-9.
BACKGROUND: Nonterpenoid and diterpenoid phenanthrenequinones (PACs) have been found in the plant kingdom. Some of them occur in plants used in traditional Chinese medicine like Tan-Shen whereas others are constituents of orchids that are popular as ornamental plants. OBJECTIVE: Case reports and our own observations in orchid nurseries suggest that some or even all of these PACs possess a distinct sensitizing potency. Occasional exposure (particularly of botanists) to field-grown orchids, as well as occupational contact with sawdust of PAC-containing tropical timbers, caused allergic contact dermatitis. However, experimental studies in guinea pigs to determine the sensitizing capacity of PACs have not been performed so far. METHODS: Guinea pigs were sensitizied by a modified Freund's complete adjuvant method with four naturally occurring and 22 synthetic PACs in order to find out which and how many substituents at the carbons of the three rings of the PAC will influence the sensitizing power of the molecule. Subsequently, the lowest unoccupied molecular orbital (LUMO) coefficients were calculated to show whether a correlation exists between chemical reactivity and sensitizing capacity. RESULTS: Sensitizing capacity was found to be strong in two PACs, moderate in eight PACs, and weak in ten PACs. Five PACs were extremely weak in sensitizing capacity, and one PAC was completely negative. Two substituents on the left-hand carbons C-7 and C-8 of ring C were shown to be responsible for a strong sensitizing capacity. One methoxy group alone or three of them, especially when localized at C-5, decreased the sensitizing capacity to moderate. Substitution with a methoxy group at C-3 and/or at C-2 of the quinonoid ring itself (ring A) led to a weak sensitizing capacity. The ortho-quinones 1,2-PAC and 9,10-PAC were also weakly sensitizing. In fact, LUMO coefficient calculations corroborated a good correlation between chemical reactivity and sensitizing capacity. CONCLUSION: Substitution with methoxy groups at C-7 and/or at C-8 of ring C of 1,4-phenanthrenequinone increases the LUMO coefficients at the 2,3 double bond of ring A and thus facilitates nucleophilic substitution of protein nitrogen or sulfur nucleophiles at this electron-deficient double bond. The four naturally occurring PACs that were investigated--cypripedin, denbinobin, annoquinone-A, and Latinone--do not fulfill these criteria and are thus only weak sensitizers. However, as-yet-unstudied phenanthrenequinones occurring in plants or trees and having no substituents at C-2 or C-3 of the quinonoid ring must be considered potentially strong allergens.
Phenolic constituents from Dalbergia cochinchinensis.[Pubmed:12932142]
J Nat Prod. 2003 Aug;66(8):1128-31.
Three new phenolic compounds (1-3), along with five known phenolics, 4'-hydroxy-2'-methoxychalcone (4), Latinone (5), dalbergiphenol (6), 7-hydroxyflavanone, and dalbergin (7), have been isolated from the stems of Dalbergia cochinchinensis. The structures of 1-3 were established by spectroscopic techniques including 1D and 2D NMR methods. The inhibitory activity against testosterone 5 alpha-reductase, which causes androgen-dependent diseases, was also examined for selected compounds.


