Lucidenic acid BCAS# 95311-95-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 95311-95-8 | SDF | Download SDF |
| PubChem ID | 102410351 | Appearance | Powder |
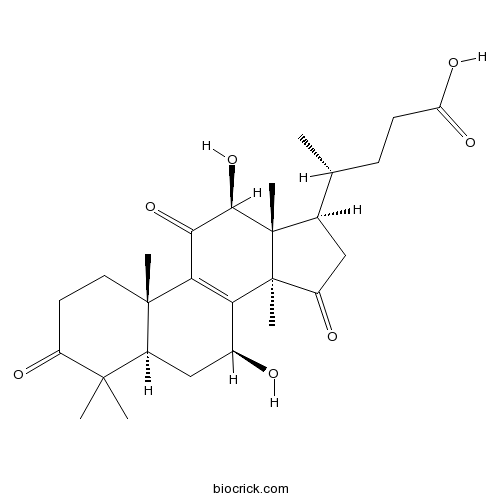
| Formula | C27H38O7 | M.Wt | 474.59 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4R)-4-[(5R,7S,10S,12S,13R,14R,17R)-7,12-dihydroxy-4,4,10,13,14-pentamethyl-3,11,15-trioxo-1,2,5,6,7,12,16,17-octahydrocyclopenta[a]phenanthren-17-yl]pentanoic acid | ||
| SMILES | CC(CCC(=O)O)C1CC(=O)C2(C1(C(C(=O)C3=C2C(CC4C3(CCC(=O)C4(C)C)C)O)O)C)C | ||
| Standard InChIKey | GYRDSOABOBCYST-HFAARYGVSA-N | ||
| Standard InChI | InChI=1S/C27H38O7/c1-13(7-8-19(31)32)14-11-18(30)27(6)20-15(28)12-16-24(2,3)17(29)9-10-25(16,4)21(20)22(33)23(34)26(14,27)5/h13-16,23,28,34H,7-12H2,1-6H3,(H,31,32)/t13-,14-,15+,16+,23-,25+,26+,27+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Lucidenic acid B shows antioxidative activity. 2. Lucidenic acid B has chemopreventive potential, it induces apoptosis in human leukemia cells via a mitochondria-mediated pathway. 3. Lucidenic acid B has anti-invasive effects, it inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1. |
| Targets | PARP | Bcl-2/Bax | Caspase | MMP(e.g.TIMP) | ERK | MEK | NF-kB | AP-1 | JNK |

Lucidenic acid B Dilution Calculator

Lucidenic acid B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1071 mL | 10.5354 mL | 21.0708 mL | 42.1416 mL | 52.677 mL |
| 5 mM | 0.4214 mL | 2.1071 mL | 4.2142 mL | 8.4283 mL | 10.5354 mL |
| 10 mM | 0.2107 mL | 1.0535 mL | 2.1071 mL | 4.2142 mL | 5.2677 mL |
| 50 mM | 0.0421 mL | 0.2107 mL | 0.4214 mL | 0.8428 mL | 1.0535 mL |
| 100 mM | 0.0211 mL | 0.1054 mL | 0.2107 mL | 0.4214 mL | 0.5268 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Toringin
Catalog No.:BCN8606
CAS No.:1329-10-8
- Saikosaponin I
Catalog No.:BCN8605
CAS No.:103629-71-6
- Uvarigranol C
Catalog No.:BCN8604
CAS No.:172104-04-0
- 9-Methymyrrhone
Catalog No.:BCN8603
CAS No.:1809980-22-0
- Asperosaponin IV
Catalog No.:BCN8602
CAS No.:126778-93-6
- Rubipodanone A
Catalog No.:BCN8601
CAS No.:2170211-22-8
- Astraganoside
Catalog No.:BCN8600
CAS No.:1011711-05-9
- Isoastragaloside IV
Catalog No.:BCN8599
CAS No.:136033-55-1
- Eriosematin
Catalog No.:BCN8598
CAS No.:168010-17-1
- Licoflavone A
Catalog No.:BCN8597
CAS No.:61153-77-3
- Dehydronuciferine
Catalog No.:BCN8596
CAS No.:7630-74-2
- Ophiopogonoside A
Catalog No.:BCN8595
CAS No.:791849-22-4
- Cnidicin
Catalog No.:BCN8608
CAS No.:14348-21-1
- Gambogellic acid
Catalog No.:BCN8609
CAS No.:173867-04-4
- Protodeltonin
Catalog No.:BCN8611
CAS No.:94992-08-2
- Licoarylcoumarin
Catalog No.:BCN8612
CAS No.:125709-31-1
- Pratensein 7-O-glucopyranoside
Catalog No.:BCN8613
CAS No.:36191-03-4
- Plantainoside D
Catalog No.:BCN8614
CAS No.:147331-98-4
- Suspenoidside B
Catalog No.:BCN8615
CAS No.:2161432-08-0
- 7beta-Hydroxybufalin
Catalog No.:BCN8616
CAS No.:20143-97-9
- 6'-O-Cinnamoylharpagide
Catalog No.:BCN8617
CAS No.:1245572-24-0
- 3,4,4a,9,10,10a-heexahydro-8-hydroxy-1-(hydroxymethyl)-1,4a-dimethyl-7-(1-methylethyl)-phenanthrene-2(1H)-one
Catalog No.:BCN8618
CAS No.:262599-12-2
- Sargentol
Catalog No.:BCN8619
CAS No.:623928-18-7
- Onjisaponin Z
Catalog No.:BCN8620
CAS No.:1078708-72-1
The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain.[Pubmed:17979098]
Mol Nutr Food Res. 2007 Dec;51(12):1472-7.
Ganoderma lucidum is a well-known mushroom with various pharmacological effects that has been used for health and longevity purposes. The objective of this study was to investigate the anti-invasive effect of lucidenic acids isolated from a new G. lucidum strain (YK-02) against human hepatoma carcinoma (HepG(2)) cells. Triterpenoid components in the ethanol extract of G. lucidum (YK-02) were separated by means of a semi-preparative RP HPLC. Four major peaks were separated and crystallized from triterpenoids fraction, and were identified as lucidenic acids A, B, C, and N according to their spectroscopic values of (1)H NMR and MS. Treatment of the lucidenic acids (50 microM) in the presence of 200 nM phorbol 12-myristate 13-acetate (PMA) after 24 h of incubation all resulted in significant inhibitory effects on PMA-induced MMP-9 activity and invasion of HepG(2 )cells. The results indicate that the lucidenic acids isolated from G. lucidum (YK-02) are anti-invasive bioactive components on hepatoma cells.
Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1.[Pubmed:18024477]
Carcinogenesis. 2008 Jan;29(1):147-56.
Ganoderma lucidum has been reported to be associated with suppressed motility, invasion and metastasis of several types of cancers, but its mechanism of action remains unclear. In our previous study, lucidenic acids A, B, C and N were isolated from a new strain of G.lucidum and all of them were found to have potential anti-invasive activity on phorbol-12-myristate-13-acetate (PMA)-induced HepG(2) cells by suppressing the matrix metalloproteinase (MMP)-9 activity. Here, the Lucidenic acid B (LAB) was used to explore its mechanisms underlying MMP-9 expression of HepG(2) cells. The results showed that the LAB suppressed PMA-induced MMP-9 activity in a dose-dependent transcriptional level. The suppression of PMA-induced MMP-9 expression of HepG(2) cells by LAB was through inactivating phosphorylation of extracellular signal-regulated kinase (ERK) 1/2. The treatment of mitogen-activated protein kinase kinase (MEK) inhibitors (PD98059 and U0126) and LAB to HepG(2) cells could result in a synergistic reduction on the MMP-9 expression along with an inhibition on cell invasion. Moreover, LAB also strongly inhibited PMA-stimulated nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) DNA-binding activities of HepG(2) cells in dose-dependent manners. A dose-dependent inhibition on protein levels of NF-kappaB, c-Jun and c-Fos in nuclear by LAB treatment was further observed. In conclusion, we demonstrated that the anti-invasive effects of the LAB on the PMA-induced HepG(2) cells might be through inhibiting the phosphorylation of ERK1/2 and reducing AP-1 and NF-kappaB DNA-binding activities, leading to downregulation of MMP-9 expression.
Triterpene antioxidants from ganoderma lucidum.[Pubmed:10479768]
Phytother Res. 1999 Sep;13(6):529-31.
Ganoderma lucidum was studied for its antioxidative activity by bioassay guided isolation in conjunction with in vitro tests. The powdered crude drug was treated with boiling water and the aqueous extract (Ex1) was further separated to obtain terpene and polysaccharide fractions. The two fractions and Ex1 were screened for their antioxidative effect against pyrogallol induced erythrocyte membrane oxidation and Fe (II)-ascorbic acid induced lipid peroxidation. All tested samples showed antioxidative activities in a dose dependent manner and the terpene fraction was found to possess the highest effect compared with the others. Chemical isolation of the terpene fraction resulted in the detection of ganoderic acids A, B, C and D, Lucidenic acid B and ganodermanontriol as major ingredients.
Lucidenic acid B induces apoptosis in human leukemia cells via a mitochondria-mediated pathway.[Pubmed:18481862]
J Agric Food Chem. 2008 Jun 11;56(11):3973-80.
Ganoderma lucidum is known as a medicinal mushroom used in traditional Chinese medicine. In the present study, the effect of lucidenic acids (A, B, C, and N) isolated from a new G. lucidum (YK-02) on induction of cell apoptosis and the apoptotic pathway in HL-60 cells were investigated. The results demonstrated that lucidenic acids decreased cell population growth of HL-60 cells, assessed with the MTT assay. The cell cycle assay indicated that treatment of HL-60 cells with lucidenic acid A, C, and N caused cell cycle arrest in the G 1 phase. Lucidenic acid B (LAB) did not affect the cell cycle profile; however, it increased the number of early and late apoptotic cells but not necrotic cells. Treatment of HL-60 cells with LAB caused loss of mitochondria membrane potential. Moreover, the ratio of expression levels of pro- and antiapoptotic Bcl-2 family members was changed by LAB treatment. LAB-induced apoptosis involved release of mitochondria cytochrome c and subsequently induced the activation of caspase-9 and caspase-3, which were followed by cleavage of poly(ADP-ribose) polymerase (PARP). Pretreatment with a general caspase-9 inhibitor (Z-LEHD-FMK) and caspase-3 inhibitor (Z-DEVD-FMK) prevented LAB from inhibiting cell viability in HL-60 cells. Our finding may be critical to the chemopreventive potential of Lucidenic acid B.


