Luteolin-3',7-di-O-glucosideCAS# 257-724-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 257-724-7 | SDF | Download SDF |
| PubChem ID | 5490298 | Appearance | Powder |
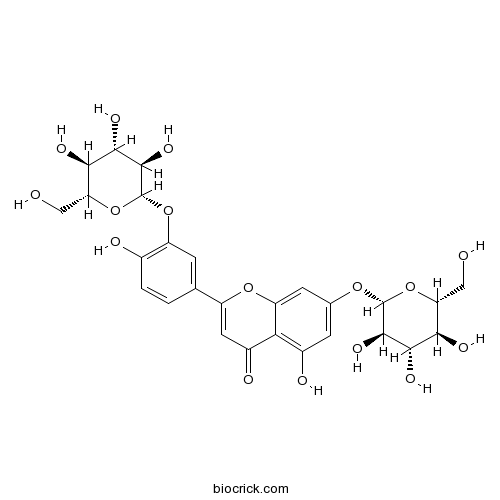
| Formula | C27H30O16 | M.Wt | 610.52 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-2-[4-hydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | ||
| SMILES | C1=CC(=C(C=C1C2=CC(=O)C3=C(C=C(C=C3O2)OC4C(C(C(C(O4)CO)O)O)O)O)OC5C(C(C(C(O5)CO)O)O)O)O | ||
| Standard InChIKey | BISZYPSIZGKOFA-IPOZFMEPSA-N | ||
| Standard InChI | InChI=1S/C27H30O16/c28-7-17-20(33)22(35)24(37)26(42-17)39-10-4-12(31)19-13(32)6-14(40-16(19)5-10)9-1-2-11(30)15(3-9)41-27-25(38)23(36)21(34)18(8-29)43-27/h1-6,17-18,20-31,33-38H,7-8H2/t17-,18-,20-,21-,22+,23+,24-,25-,26-,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Luteolin-3',7-di-O-glucoside has anti-ulcer and antioxidant activities. |
| In vitro | Antioxidant activities and quali-quantitative analysis of different Smallanthus sonchifolius [(Poepp. and Endl.) H. Robinson] landrace extracts.[Pubmed: 25533266 ]Nat Prod Res. 2015;29(17):1673-7.Five landraces of Smallanthus sonchifolius [(Poepp. and Endl.) H. Robinson], known as yacon, were investigated in total phenolic content, antioxidant activity and chemical composition of ethanol extracts (EEs) and decoction extracts (DEs). |
| In vivo | Antiulcer activity of the ethanolic extract and ethyl acetate fraction of the leaves of Markhamia tomentosa in rats.[Pubmed: 25240588]J Ethnopharmacol. 2014 Nov 18;157:1-6.The leaves of Markhamia tomentosa (Benth.) K. Schum. Ex Engl. (Bignoniaceae) are used traditionally in the treatment of skin afflictions, sores, ulcers and inflammation. The aim of the study was to investigate the antiulcer activity of the crude ethanolic extract from the leaves of Markhamia tomentosa, determine the active fraction(s) of the extract and identify the chemical constituents in the active fraction by LC-MS.
|

Luteolin-3',7-di-O-glucoside Dilution Calculator

Luteolin-3',7-di-O-glucoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6379 mL | 8.1897 mL | 16.3795 mL | 32.759 mL | 40.9487 mL |
| 5 mM | 0.3276 mL | 1.6379 mL | 3.2759 mL | 6.5518 mL | 8.1897 mL |
| 10 mM | 0.1638 mL | 0.819 mL | 1.6379 mL | 3.2759 mL | 4.0949 mL |
| 50 mM | 0.0328 mL | 0.1638 mL | 0.3276 mL | 0.6552 mL | 0.819 mL |
| 100 mM | 0.0164 mL | 0.0819 mL | 0.1638 mL | 0.3276 mL | 0.4095 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isosilybin B
Catalog No.:BCN6764
CAS No.:142796-22-3
- Arecaidine
Catalog No.:BCN8282
CAS No.:499-04-7
- Xanthoangelol
Catalog No.:BCN8741
CAS No.:62949-76-2
- Trillikamtoside Q
Catalog No.:BCN8195
CAS No.:2098642-70-5
- 2''-O-acetylsaikosaponin A
Catalog No.:BCN8736
CAS No.:102934-42-9
- (R)-alpha-methyltryptamine
Catalog No.:BCN8160
CAS No.:7795-52-0
- Quercetin 3-O-(6''-galloyl)-beta-D-glucopyranoside
Catalog No.:BCN8742
CAS No.:56316-75-7
- Scutellarein-7-O-glucoside
Catalog No.:BCN8099
CAS No.:26046-94-6
- Steviol-19-O-Glucoside
Catalog No.:BCN6373
CAS No.:1185737-16-9
- (-)-Epiafzelechin 3-O-gallate
Catalog No.:BCN8740
CAS No.:108907-43-3
- 8-Hydroxypinoresinol diglucoside
Catalog No.:BCN6368
CAS No.:112747-99-6
- Caraganaphenol A
Catalog No.:BCN8731
CAS No.:174916-31-5
- Isosilybin A
Catalog No.:BCN6369
CAS No.:142796-21-2
- Cistantubuloside C1
Catalog No.:BCN6362
CAS No.:620632-36-2
- Niga-ichigoside F1
Catalog No.:BCN8356
CAS No.:95262-48-9
- 1,3,6-Trihydroxy-2-methylanthraquinone 3-O-alpha-L-rhamnosyl-(1->2)-beta-D-glucoside
Catalog No.:BCN8732
CAS No.:87686-88-2
- L-Fucitol
Catalog No.:BCN8464
CAS No.:13074-06-1
- Ganoderic acid R
Catalog No.:BCN8489
CAS No.:103963-39-9
- Tessaric acid
Catalog No.:BCN8743
CAS No.:58142-10-2
- Quercetin 3-Caffeylrobinobioside
Catalog No.:BCN8744
CAS No.:957110-26-8
- Momordicine II
Catalog No.:BCN8745
CAS No.:91590-75-9
- Polygalasaponin XXXV
Catalog No.:BCN8746
CAS No.:184479-28-5
- Marginatoxin
Catalog No.:BCN8747
CAS No.:1422536-56-8
- Leachianol G
Catalog No.:BCN8748
CAS No.:164204-62-0
Antiulcer activity of the ethanolic extract and ethyl acetate fraction of the leaves of Markhamia tomentosa in rats.[Pubmed:25240588]
J Ethnopharmacol. 2014 Nov 18;157:1-6.
ETHNOPHARMACOLOGICAL RELEVANCE: The leaves of Markhamia tomentosa (Benth.) K. Schum. Ex Engl. (Bignoniaceae) are used traditionally in the treatment of skin afflictions, sores, ulcers and inflammation. The aim of the study was to investigate the antiulcer activity of the crude ethanolic extract from the leaves of Markhamia tomentosa, determine the active fraction(s) of the extract and identify the chemical constituents in the active fraction by LC-MS. MATERIALS AND METHODS: The antiulcer activity of the crude extract (50, 100 and 150mg/kg, p.o.) was evaluated in ethanol and indomethacin-induced models while the solvent fractions (150mg/kg) were screened using ethanol-induced gastric lesions in rats. Furthermore, anti-ulcer activity of the active fraction (50, 100 and 150mg/kg, p.o.) was performed using indomethacin and pylorus ligation models. Parameters such as gastric volume, pH and acidity were determined in the pylorus ligation model. LC-ESI-MS analysis was used to identify the components in the active fraction. RESULTS: The extract at the dose of 50, 100 and 150mg/kg caused a significant (p<0.05) dose-dependent inhibition of ulcer in the ethanol and indomethacin-induced ulcer models, respectively. The ethyl acetate (EtOAc) fraction showed the most potent antiulcer activity from all the fractions tested. This fraction produced 72% and 92% inhibition of indomethacin and pylorus-induced ulcer at a dose of 150mg/kg respectively. Acteoside, luteolin, luteolin-7-rutinoside, Luteolin-3',7-di-O-glucoside, carnosol, dilapachone, tormentic acid, oxo-pomolic acid and ajugol were detected in the EtOAc fraction. CONCLUSION: Our data provide a rational base for the folkloric use of Markhamia tormentosa in the treatment of ulcers.
Simultaneous determination of glucuronic acid and sulfuric acid conjugated metabolites of daidzein and genistein in human plasma by high-performance liquid chromatography.[Pubmed:20149762]
J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Mar 1;878(7-8):628-36.
Isoflavone aglycones daidzein (Dein) and genistein (Gein) are mainly present as glucuronides and sulfates in human plasma, and small amounts of the intact aglycones are also detected. In the present study, we have developed a high-performance liquid chromatography (HPLC)-UV-diode-array detector (DAD) method for the determination of intact 16 metabolites of Dein and Gein in plasma, especially focusing on highly polar conjugated metabolites at both 4' and 7 positions on the isoflavone ring with glucuronic acid and/or sulfuric acid (7-glucuronide-4'-sulfates and 4',7-diglucuronides). Luteolin-3',7-di-O-glucoside was used as an internal standard. Solid-phase extraction was performed on an Oasis HLB cartridge (60 mg, 3 cm(3)) with a recovery of >ca. 80%. The HPLC assay was performed on a Hydrosphere C18 column (100 mm x 4.6 mm I.D., particle size 3 microm). The mobile phase consisted of a mixture of 10 mM ammonium acetate solution and acetonitrile run under gradient mode at a flow rate of 1.5 ml/min. The UV detection wavelength was set at 250 nm. For UV spectral analysis, the diode-array detection wavelength was set at 220-360 nm. All HPLC analyses were performed at 45 degrees C. Each calibration for the determination of 16 metabolites gave a linear signal (r>0.997) over a concentration range of 5-5000 ng/ml. The lower limits of quantification of these metabolites were 21.1-23.4 ng/ml and the lower limits of detection were 7.9-9.4 ng/ml. This method was used in a preliminary experiment to determine the plasma concentration of intact 16 metabolites after oral administration of kinako (baked soybean powder) to a healthy volunteer. The present HPLC-UV-DAD method should be useful for the metabolic and pharmacokinetic investigations of isoflavones in humans.


