Microgrewiapine ACAS# 1420777-30-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1420777-30-5 | SDF | Download SDF |
| PubChem ID | 71576920 | Appearance | Powder |
| Formula | C17H29NO | M.Wt | 263.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
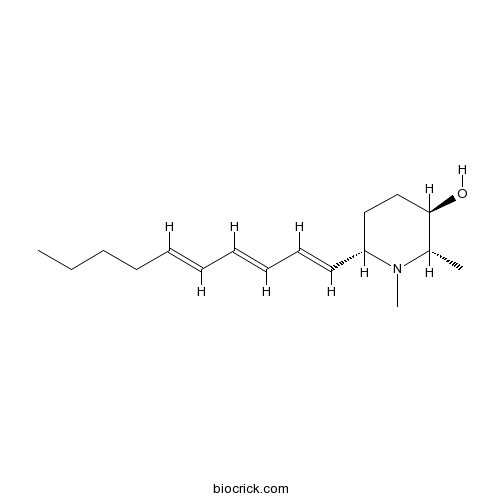
| Chemical Name | (2S,3R,6S)-6-[(1E,3E,5E)-deca-1,3,5-trienyl]-1,2-dimethylpiperidin-3-ol | ||
| SMILES | CCCCC=CC=CC=CC1CCC(C(N1C)C)O | ||
| Standard InChIKey | ZBJGGLXQNXXXRO-VCZRHBNQSA-N | ||
| Standard InChI | InChI=1S/C17H29NO/c1-4-5-6-7-8-9-10-11-12-16-13-14-17(19)15(2)18(16)3/h7-12,15-17,19H,4-6,13-14H2,1-3H3/b8-7+,10-9+,12-11+/t15-,16+,17+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Microgrewiapine A is a selective cytotoxic agent for colon cancer cells over normal colon cells and to exhibit nicotinic receptor antagonistic activity for both the hα3β4 and hα4β2 receptor subtypes. |
| Targets | hα3β4 receptor | hα4β2 receptor |
| In vitro | Alkaloids from Microcos paniculata with cytotoxic and nicotinic receptor antagonistic activities.[Pubmed: 23327794]J Nat Prod. 2013 Feb 22;76(2):243-9.Microcos paniculata is a large shrub or small tree that grows in several countries in South and Southeast Asia.
|
| Structure Identification | Chem Biodivers. 2017 Dec;14(12).Quick Identification of Piperidine Alkaloid from Roots of Grewia nervosa and Their Glucosidase Inhibitory Activity.[Pubmed: 29044865 ]
Grewia nervosa is a herbal plant used in traditional medicine for different purposes.
|

Microgrewiapine A Dilution Calculator

Microgrewiapine A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7965 mL | 18.9825 mL | 37.9651 mL | 75.9301 mL | 94.9127 mL |
| 5 mM | 0.7593 mL | 3.7965 mL | 7.593 mL | 15.186 mL | 18.9825 mL |
| 10 mM | 0.3797 mL | 1.8983 mL | 3.7965 mL | 7.593 mL | 9.4913 mL |
| 50 mM | 0.0759 mL | 0.3797 mL | 0.7593 mL | 1.5186 mL | 1.8983 mL |
| 100 mM | 0.038 mL | 0.1898 mL | 0.3797 mL | 0.7593 mL | 0.9491 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ent-16beta,17-dihydroxy-9(11)-kauren-19-oic acid
Catalog No.:BCN8759
CAS No.:55483-24-4
- Torosachrysone 8-O-beta-gentiobioside
Catalog No.:BCN8758
CAS No.:94356-13-5
- Ampelopsin G
Catalog No.:BCN8757
CAS No.:151487-09-1
- Yuanhuanin
Catalog No.:BCN8756
CAS No.:83133-14-6
- Polygalasaponin E
Catalog No.:BCN8754
CAS No.:882664-72-4
- Leachianol F
Catalog No.:BCN8753
CAS No.:164123-50-6
- Pueroside A
Catalog No.:BCN8752
CAS No.:100692-52-2
- Momordicine V
Catalog No.:BCN8751
CAS No.:1012315-36-4
- Cassiaglycoside II
Catalog No.:BCN8750
CAS No.:2241081-56-9
- Atractylochromene
Catalog No.:BCN8749
CAS No.:203443-33-8
- Leachianol G
Catalog No.:BCN8748
CAS No.:164204-62-0
- Marginatoxin
Catalog No.:BCN8747
CAS No.:1422536-56-8
- Rhamnocitrin 3-glucoside
Catalog No.:BCN8761
CAS No.:41545-37-3
- (1,5E,11E)-tridecatriene-7,9-diyne-3,4-diacetate
Catalog No.:BCN8762
CAS No.:201012-14-8
- 3,7,23,24-tetrahydroxycucurbita-5,25-dien-19-al
Catalog No.:BCN8763
CAS No.:1446447-97-7
- Orientalide
Catalog No.:BCN8764
CAS No.:72704-05-3
- Rhamnocitrin 3-apiosyl-(1→2)-glucoside
Catalog No.:BCN8765
CAS No.:148031-68-9
- Daidzein-4'-glucoside
Catalog No.:BCN8766
CAS No.:58970-69-7
- Formononetin-8-C-beta-D-apiofuranosyl-(1->6)-O-beta-D-glucopyranoside
Catalog No.:BCN8767
CAS No.:1147858-78-3
- Pueroside B
Catalog No.:BCN8768
CAS No.:100692-54-4
- Momordicoside X
Catalog No.:BCN8769
CAS No.:1333321-50-8
- Pueroside C
Catalog No.:BCN8770
CAS No.:112343-16-5
- 1,7-Diphenyl-5-hydroxy-4,6-hepten-3-one
Catalog No.:BCN8771
CAS No.:87095-77-0
- Genistein 7-O-beta-D-glucopyranoside-4'-O-[alpha-L-rhamnopyranosyl-(1->2)-beta-D-glucopyranoside]
Catalog No.:BCN8772
CAS No.:70404-42-1
Total Synthesis and Absolute Stereochemical Assignment of Microgrewiapine A and Its Stereoisomers.[Pubmed:30406652]
J Org Chem. 2019 Jan 4;84(1):94-103.
Total synthesis of both enantiomers of (-)-(2 S,3 R,6 S)- and (+)-(2 R,3 S,6 R)-Microgrewiapine A along with (+)-microcosamine A and (-)-6- epi-Microgrewiapine A from chiral 1-(alpha-methylbenzyl)-aziridine-2-carboxylate was accomplished for the first time. Key steps involved in this synthesis include one-pot reductive ring-opening of aziridine, debenzylation, intramolecular N-alkylation to obtain the key piperidine ring, and Julia-Kociensky olefination. The absolute configuration of natural Microgrewiapine A is assigned as (+)-(2 R,3 S,6 R), which is opposite to the originally proposed structure by comparing optical rotation data of both synthetic enantiomers.
Quick Identification of Piperidine Alkaloid from Roots of Grewia nervosa and Their Glucosidase Inhibitory Activity.[Pubmed:29044865]
Chem Biodivers. 2017 Dec;14(12).
Grewia nervosa is a herbal plant used in traditional medicine for different purposes. Bioassay-guided chemical fractionation of G. nervosa roots resulted in an identification of two known and one new compound, namely Microgrewiapine A, homomicrogrewiapine, and N-methylmicrocosamine, respectively. Their structures were determined using combination of LC/HR-MS, (1) H-NMR, and IR spectral analyses and followed by comparison with those reported in the literature. The problematic separation of these alkaloids on traditional column chromatography (Silica gel, Octadecyl silane, Sephadex) was resolved by using HPLC. Structurally similar compounds from the piperidine family have been characterized by using HR-MS analysis in combination with NMR data of crude samples. The major constituent i.e. N-methylmicrocosamine isolated from the butanol fraction of methanol root extract (MRE) was found to possess the dose dependent alpha-glucosidase inhibition activity with an IC50 value of 53.40 mum. Furthermore, N-methylmicrocosamine showed maximum alpha-glucosidase inhibition of 97.48 +/- 0.7% at 107.5 mum, which is approximately 1.3 x 10(3) fold higher than the activity shown by acarbose (97.72% inhibition at 61.95 mm), a standard anti-diabetic drug available commercially. This work also reports the in vitro alpha-glucosidase inhibitory activity of the major alkaloids isolated from G. nervosa for the first time.
Alkaloids from Microcos paniculata with cytotoxic and nicotinic receptor antagonistic activities.[Pubmed:23327794]
J Nat Prod. 2013 Feb 22;76(2):243-9.
Microcos paniculata is a large shrub or small tree that grows in several countries in South and Southeast Asia. In the present study, three new piperidine alkaloids, microgrewiapines A-C (1-3), as well as three known compounds, inclusive of microcosamine A (4), 7'-(3',4'-dihydroxyphenyl)-N-[4-methoxyphenyl)ethyl]propenamide (5), and liriodenine (6), were isolated from cytotoxic fractions of the separate chloroform-soluble extracts of the stem bark, branches, and leaves of M. paniculata. Compounds 1-6 and 1a (Microgrewiapine A 3-acetate) showed a range of cytotoxicity values against the HT-29 human colon cancer cell line. When evaluated for their effects on human alpha3beta4 or alpha4beta2 nicotinic acetylcholine receptors (nAChRs), several of these compounds were shown to be active as nAChR antagonists. As a result of this study, Microgrewiapine A (1) was found to be a selective cytotoxic agent for colon cancer cells over normal colon cells and to exhibit nicotinic receptor antagonistic activity for both the halpha3beta4 and halpha4beta2 receptor subtypes.


