Murrayafoline ACAS# 4532-33-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4532-33-6 | SDF | Download SDF |
| PubChem ID | 375150 | Appearance | Oil |
| Formula | C14H13NO | M.Wt | 211.26 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
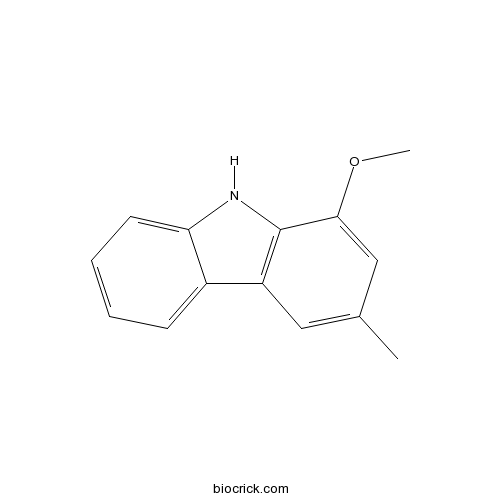
| Chemical Name | 1-methoxy-3-methyl-9H-carbazole | ||
| SMILES | CC1=CC2=C(C(=C1)OC)NC3=CC=CC=C32 | ||
| Standard InChIKey | HDETUOZJFUNSKG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H13NO/c1-9-7-11-10-5-3-4-6-12(10)15-14(11)13(8-9)16-2/h3-8,15H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Murrayafoline A Dilution Calculator

Murrayafoline A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7335 mL | 23.6675 mL | 47.335 mL | 94.6701 mL | 118.3376 mL |
| 5 mM | 0.9467 mL | 4.7335 mL | 9.467 mL | 18.934 mL | 23.6675 mL |
| 10 mM | 0.4734 mL | 2.3668 mL | 4.7335 mL | 9.467 mL | 11.8338 mL |
| 50 mM | 0.0947 mL | 0.4734 mL | 0.9467 mL | 1.8934 mL | 2.3668 mL |
| 100 mM | 0.0473 mL | 0.2367 mL | 0.4734 mL | 0.9467 mL | 1.1834 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Thymyl 2-methylbutyrate
Catalog No.:BCN9344
CAS No.:69844-32-2
- Methyl ganoderenate D
Catalog No.:BCN9343
CAS No.:748136-03-0
- Methyl lucidenate N
Catalog No.:BCN9342
CAS No.:1276655-49-2
- Cadambine
Catalog No.:BCN9341
CAS No.:54422-49-0
- 8,9-Dehydro-7,9-diisobutyryloxythymol
Catalog No.:BCN9340
CAS No.:22518-03-2
- Hancolupenone
Catalog No.:BCN9339
CAS No.:132746-04-4
- Phochinenin G
Catalog No.:BCN9338
CAS No.:1070883-75-8
- Jasnervoside C
Catalog No.:BCN9337
CAS No.:1622337-36-3
- 1-Methyleffusol
Catalog No.:BCN9336
CAS No.:144106-78-5
- Thymol isobutyrate
Catalog No.:BCN9335
CAS No.:5451-67-2
- Aureusidin
Catalog No.:BCN9334
CAS No.:38216-54-5
- Sampsone B
Catalog No.:BCN9333
CAS No.:1309125-17-4
- Phochinenin I
Catalog No.:BCN9346
CAS No.:1070883-77-0
- 2α,3α,24-Trihydroxyursa-12,20(30)-dien-28-oic acid
Catalog No.:BCN9347
CAS No.:341503-22-8
- 8-Hydroxythymol
Catalog No.:BCN9348
CAS No.:4478-33-5
- Visartiside E
Catalog No.:BCN9349
CAS No.:1212005-08-7
- Girinimbine
Catalog No.:BCN9350
CAS No.:23095-44-5
- Benzyl [5-O-benzoyl-β-D-apiofuranosyl(1→2)]-β-D-glucopyranoside
Catalog No.:BCN9351
CAS No.:1097040-08-8
- Pomegralignan
Catalog No.:BCN9352
CAS No.:1562492-32-3
- Methyl lucidenate D
Catalog No.:BCN9353
CAS No.:98665-09-9
- 1-[2-Hydroxy-4-(hydroxymethyl)phenyl]ethanone
Catalog No.:BCN9354
CAS No.:22518-00-9
- Methyl lucidenate A
Catalog No.:BCN9355
CAS No.:105742-79-8
- 3-Hydroxyperillaldehyde
Catalog No.:BCN9356
CAS No.:1932348-11-2
- Convallagenin B
Catalog No.:BCN9357
CAS No.:17934-59-7
[Chemical constituents from stems and leaves of Clausena emarginata].[Pubmed:31355567]
Zhongguo Zhong Yao Za Zhi. 2019 May;44(10):2096-2101.
The chemical constituents from the stems and leaves of Clausena emarginata were separated and purified by column chromatographies on silica gel,ODS,Sephadex LH-20,and PR-HPLC. The structures of the isolated compounds were identified on the basis of physicochemical properties and spectroscopic analysis,as well as comparisons with the data reported in the literature. Sixteen compounds were isolated from the 90% ethanol extract of the stems and leaves of C. emarginata,which were identified as siamenol( 1),murrastanine A( 2),3-formyl-1,6-dimethoxycarbazole( 3),3-methoxymethylcarbazole( 4),3-methylcarbazole( 5),Murrayafoline A( 6),3-formylcarbazole( 7),3-formyl-1-hydroxycarbazole( 8),3-formyl-6-methoxycarbazole( 9),murrayanine( 10),murrayacine( 11),girinimbine( 12),nordentatin( 13),chalepin( 14),8-hydroxy-6-methoxy-3-pentylisocoumarin( 15) and ethyl orsellinate( 16). Compounds 1-4,14-16 were isolated from C. emarginata for the first time. Among them,compounds 1,2,15 and 16 were isolated from the genus Clausena for the first time. All isolated compounds were evaluated for their cytotoxic activities against five human cancer cell lines: HL-60,SMMC-7721,A-549,MCF-7 and SW480 in vitro. Compounds 12 and 14 showed significant inhibitory effects against various human cancer cell lines with IC_(50) values comparable to those of doxorubicin.
Functional, electrophysiological and molecular docking analysis of the modulation of Cav 1.2 channels in rat vascular myocytes by murrayafoline A.[Pubmed:26493241]
Br J Pharmacol. 2016 Jan;173(2):292-304.
BACKGROUND AND PURPOSE: The carbazole alkaloid Murrayafoline A (MuA) enhances contractility and the Ca(2+) currents carried by the Cav 1.2 channels [ICa1.2 ] of rat cardiomyocytes. As only few drugs stimulate ICa1.2 , this study was designed to analyse the effects of MuA on vascular Cav 1.2 channels. EXPERIMENTAL APPROACH: Vascular activity was assessed on rat aorta rings mounted in organ baths. Cav 1.2 Ba(2+) current [IBa1.2 ] was recorded in single rat aorta and tail artery myocytes by the patch-clamp technique. Docking at a 3D model of the rat, alpha1c central pore subunit of the Cav 1.2 channel was simulated in silico. KEY RESULTS: In rat aorta rings MuA, at concentrations 14.2 muM, it relaxed high K(+) depolarized rings and antagonized Bay K 8644-induced contraction. In single myocytes, MuA stimulated IBa1.2 in a concentration-dependent, bell-shaped manner; stimulation was stable, incompletely reversible upon drug washout and accompanied by a leftward shift of the voltage-dependent activation curve. MuA docked at the alpha1C subunit central pore differently from nifedipine and Bay K 8644, although apparently interacting with the same amino acids of the pocket. Neither Bay K 8644-induced stimulation nor nifedipine-induced block of IBa1.2 was modified by MuA. CONCLUSIONS AND IMPLICATIONS: Murrayafoline A is a naturally occurring vasoactive agent able to modulate Cav 1.2 channels and dock at the alpha1C subunit central pore in a manner that differed from that of dihydropyridines.
Murrayafoline A Induces a G0/G1-Phase Arrest in Platelet-Derived Growth Factor-Stimulated Vascular Smooth Muscle Cells.[Pubmed:26330754]
Korean J Physiol Pharmacol. 2015 Sep;19(5):421-6.
The increased potential for vascular smooth muscle cell (VSMC) growth is a key abnormality in the development of atherosclerosis and post-angioplasty restenosis. Abnormally high activity of platelet-derived growth factor (PDGF) is believed to play a central role in the etiology of these pathophysiological situations. Here, we investigated the anti-proliferative effects and possible mechanism(s) of Murrayafoline A, a carbazole alkaloid isolated from Glycosmis stenocarpa Guillamin (Rutaceae), on PDGF-BB-stimulated VSMCs. Murrayafoline A inhibited the PDGF-BB-stimulated proliferation of VSMCs in a concentration-dependent manner, as measured using a non-radioactive colorimetric WST-1 assay and direct cell counting. Furthermore, Murrayafoline A suppressed the PDGF-BB-stimulated progression through G0/G1 to S phase of the cell cycle, as measured by [(3)H]-thymidine incorporation assay and cell cycle progression analysis. This anti-proliferative action of Murrayafoline A, arresting cell cycle progression at G0/G1 phase in PDGF-BB-stimulated VSMCs, was mediated via down-regulation of the expression of cyclin D1, cyclin E, cyclin-dependent kinase (CDK)2, CDK4, and proliferating cell nuclear antigen (PCNA), and the phosphorylation of retinoblastoma protein (pRb). These results indicate that Murrayafoline A may be useful in preventing the progression of vascular complications such as restenosis after percutaneous transluminal coronary angioplasty and atherosclerosis.
Sensitization of cardiac Ca(2)(+) release sites by protein kinase C signaling: evidence from action of murrayafoline A.[Pubmed:25095987]
Pflugers Arch. 2015 Jul;467(7):1607-1621.
In the present study, we explored the effects of a plant alkaloid compound, 1-methoxy-3-methylcarbazole (Murrayafoline A, Mu-A), on focal and global Ca(2+) signaling, and the underlying cellular mechanisms. Rapid two-dimensional confocal Ca(2+) imaging and image analysis were used to measure Ca(2+) signals in rat ventricular myocytes. Application of Mu-A (10-100 muM) significantly enhanced the magnitude and rate of Ca(2+) release on depolarization with no change in Ca(2+) transient decay. Focal Ca(2+) release events (Ca(2+) sparks) occurred more often, and their duration and size were greater after the application of Mu-A. In addition, sarcoplasmic reticulum (SR) Ca(2+) loading and fractional release were increased by exposure to Mu-A. All these effects reached steady state within 2-3 min after Mu-A application. The higher occurrence of Ca(2+) sparks in the presence of Mu-A was resistant to SR Ca(2+) clamping, removal of extracellular Ca(2+) and Na(+), and blockade of either protein kinase A, Ca(2+)/calmodulin-dependent protein kinase II, phospholipase C, or inositol 1,4,5-trisphosphate receptors, but it was abolished by the inhibition of protein kinase C (PKC). SR Ca(2+) clamping prevented the Mu-A-induced Ca(2+) spark prolongation and enlargement. The Mu-A-induced enhancement of Ca(2+) transients was also eliminated by PKC blockade. Mu-A enhanced PKC activity in vitro. These results suggest that Mu-A may increase spark occurrence via its direct enhancement of PKC activity and subsequent sensitization of ryanodine receptor clusters and that this mechanism, as well as increased SR Ca(2+) loading, may partly explain larger and more rapid global Ca(2+) releases in the presence of Mu-A during depolarization.
Iodine-catalyzed aromatization of tetrahydrocarbazoles and its utility in the synthesis of glycozoline and murrayafoline A: a combined experimental and computational investigation.[Pubmed:24938996]
Org Biomol Chem. 2014 Jul 21;12(27):4832-6.
A new protocol for the aromatization of tetrahydrocarbazoles has been achieved using a catalytic amount of iodine, giving high yields. The role of iodine in the aromatization has been explained by DFT, and its wide scope is extended to the total synthesis of glycozoline and Murrayafoline A. This method has proven to be tolerant of a broad range of functional groups.
Copper-catalyzed N-H insertion and oxidative aromatization cascade: facile synthesis of 2-arylaminophenols.[Pubmed:24771474]
Chem Asian J. 2014 Jun;9(6):1539-42.
A copper-catalyzed cascade reaction of N-H insertion and oxidative aromatization has been developed. 2-Arylaminophenols have been prepared in moderate to high yields from the diazo substrates. Moreover, this newly established methodology allows efficient access to natural 1-oxygenated carbazole alkaloids, such as glycozolicine and Murrayafoline A.
Synthesis of novel derivatives of murrayafoline A and their inhibitory effect on LPS-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells.[Pubmed:23564015]
Arch Pharm Res. 2013 Jul;36(7):832-9.
A series of N-substituted-1,2,3-triazole Murrayafoline A derivatives were successfully synthesized using click azide-alkyne Huisgen cycloaddition reaction between 1-methoxy-3-methyl-9-(3-azido)-propyl-9H-carbazole and substituted alkynes. Their chemical structures were confirmed by (1)H, (13)C NMR and HR-ESI-MS spectral data. In addition, the interested effects on LPS-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells of synthetic Murrayafoline A derivatives were also investigated. Our results indicated that Murrayafoline A derivatives containing 1,2,3-triazole nucleus potentially possessed anti-inflammatory action through inhibiting production of IL-6, IL-12 p40 and TNF-alpha.
Murrayafoline A attenuates the Wnt/beta-catenin pathway by promoting the degradation of intracellular beta-catenin proteins.[Pubmed:19962966]
Biochem Biophys Res Commun. 2010 Jan 1;391(1):915-20.
Molecular lesions in Wnt/beta-catenin signaling and subsequent up-regulation of beta-catenin response transcription (CRT) occur frequently during the development of colon cancer. To identify small molecules that suppress CRT, we screened natural compounds in a cell-based assay for detection of TOPFalsh reporter activity. Murrayafoline A, a carbazole alkaloid isolated from Glycosmis stenocarpa, antagonized CRT that was stimulated by Wnt3a-conditioned medium (Wnt3a-CM) or LiCl, an inhibitor of glycogen synthase kinase-3beta (GSK-3beta), and promoted the degradation of intracellular beta-catenin without altering its N-terminal phosphorylation at the Ser33/37 residues, marking it for proteasomal degradation, or the expression of Siah-1, an E3 ubiquitin ligase. Murrayafoline A repressed the expression of cyclin D1 and c-myc, which is known beta-catenin/T cell factor (TCF)-dependent genes and thus inhibited the proliferation of various colon cancer cells. These findings indicate that Murrayafoline A may be a potential chemotherapeutic agent for use in the treatment of colon cancer.


