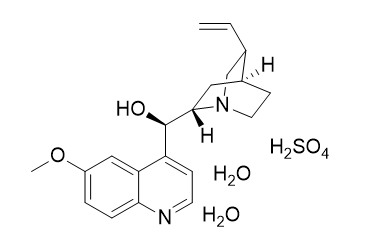
Quinine sulfate dihydrateCAS# 6119-70-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 6119-70-6 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C20H30N2O8S | M.Wt | 458.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Quinine sulfate: a pharmaceutical product as effective corrosion inhibitor for carbon steel in hydrochloric acid solution. | |||||

Quinine sulfate dihydrate Dilution Calculator

Quinine sulfate dihydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.181 mL | 10.9051 mL | 21.8103 mL | 43.6205 mL | 54.5256 mL |
| 5 mM | 0.4362 mL | 2.181 mL | 4.3621 mL | 8.7241 mL | 10.9051 mL |
| 10 mM | 0.2181 mL | 1.0905 mL | 2.181 mL | 4.3621 mL | 5.4526 mL |
| 50 mM | 0.0436 mL | 0.2181 mL | 0.4362 mL | 0.8724 mL | 1.0905 mL |
| 100 mM | 0.0218 mL | 0.1091 mL | 0.2181 mL | 0.4362 mL | 0.5453 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rhoeadine
Catalog No.:BCN9760
CAS No.:2718-25-4
- Allylcysteine
Catalog No.:BCN9759
CAS No.:21593-77-1
- Ruberythric acid
Catalog No.:BCN9758
CAS No.:152-84-1
- Octanoic acid
Catalog No.:BCN9757
CAS No.:124-07-2
- 6,7-Dihydroxyflavone
Catalog No.:BCN9756
CAS No.:38183-04-9
- 5-Methoxyflavanone
Catalog No.:BCN9755
CAS No.:55947-36-9
- Arganine B
Catalog No.:BCN9754
CAS No.:144425-21-8
- (+)-Menthofuran
Catalog No.:BCN9753
CAS No.:17957-94-7
- Benzyl acetate
Catalog No.:BCN9752
CAS No.:140-11-4
- 3',4',5,5',6,7-Hexamethoxyflavone
Catalog No.:BCN9751
CAS No.:29043-07-0
- Artemicapin C
Catalog No.:BCN9750
CAS No.:334007-19-1
- Neosalvianen
Catalog No.:BCN9749
CAS No.:790673-00-6
- 2,3-Dihydro-2-phenyl-4H-benzopyran-4-one
Catalog No.:BCN9762
CAS No.:487-26-3
- Dodeca 2E,4E,8Z,10E,Z-tetraenoic acid isobutylamide
Catalog No.:BCN9763
CAS No.:866602-52-0
- Glucofrangulin B
Catalog No.:BCN9764
CAS No.:14062-59-0
- L-(-)-Malic acid
Catalog No.:BCN9765
CAS No.:97-67-6
- (S)-(-)-Limonene
Catalog No.:BCN9766
CAS No.:5989-54-8
- Rebaudioside M
Catalog No.:BCN9767
CAS No.:1220616-44-3
- N-trans-caffeoyloctopamine
Catalog No.:BCN9768
CAS No.:1378868-10-0
- 2'-Methoxyflavone
Catalog No.:BCN9769
CAS No.:19725-47-4
- Comanthosid A
Catalog No.:BCN9770
CAS No.:70938-59-9
- (-)-Eburnamonine
Catalog No.:BCN9771
CAS No.:4880-88-0
- 4'-Methoxychalcone
Catalog No.:BCN9772
CAS No.:959-23-9
- DL-Tyrosine
Catalog No.:BCN9773
CAS No.:556-03-6
Discrimination of Isointense Bitter Stimuli in a Beer Model System.[Pubmed:32471227]
Nutrients. 2020 May 27;12(6). pii: nu12061560.
Prior work suggests humans can differentiate between bitter stimuli in water. Here, we describe three experiments that test whether beer consumers can discriminate between different bitterants in beer. In Experiment 1 (n = 51), stimuli were intensity matched; Experiments 2 and 3 were a difference from control (DFC)/check-all-that-apply (CATA) test (n = 62), and an affective test (n = 81). All used a commercial non-alcoholic beer spiked with Isolone (a hop extract), Quinine sulfate dihydrate, and sucrose octaacetate (SOA). In Experiment 1, participants rated intensities on general labeled magnitude scales (gLMS), which were analyzed via ANOVA. In Experiment 2, participants rated how different samples were from a reference of Isolone on a 7-point DFC scale, and endorsed 13 attributes in a CATA task. DFC data were analyzed via ANOVA with Dunnett's test to compare differences relative to a blind reference, and CATA data were analyzed via Cochran's Q test. In Experiment 3, liking was assessed on labeled affective magnitude scales, and samples were also ranked. Liking was analyzed via ANOVA and rankings were analyzed with a Cochran-Mantel-Haenszel test. Experiment 1 confirmed that samples were isointense. In Experiment 2, despite being isointense, both quinine (p = 0.04) and SOA (p = 0.03) were different from Isolone, but no significant effects were found for CATA descriptors (all p values > 0.16). In Experiment 3, neither liking (p = 0.16) or ranking (p = 0.49) differed. Collectively, these data confirm that individuals can discriminate perceptually distinct bitter stimuli in beer, as shown previously in water, but these differences cannot be described semantically, and they do not seem to influence hedonic assessments.
Investigation of the quinine sulfate dihydrate spectral properties and its effects on Cherenkov dosimetry.[Pubmed:31181556]
Phys Med Biol. 2019 Aug 7;64(15):155019.
Recent studies have proposed that adding quinine to water while performing Cherenkov volumetric dosimetry improves the skewed percent depth dose measurement. The aim of this study was to quantify the ability of quinine to convert directional Cherenkov emission to isotropic fluorescence and evaluate its contribution to the total emitted light. Aqueous solutions of quinine were prepared with distilled water at various concentrations (0.01-1.2 g l(-1)). The solutions were irradiated with photon beams at 6 and 23 MV. The dependence of the light produced as a function of sample concentration was studied using a spectrometer with a fixed integration time. Spectral measurements of the luminescent solution and the blank solution (distilled water only) were taken to deconvolve the Cherenkov and quinine contribution to the overall emission spectrum. Using a CCD camera, intensity profiles were obtained for the blank and the 1.00 g l(-1) solutions to compare them with the dose predicted by a treatment planning system. The luminescent intensity of the samples was found to follow a logarithmic trend as a function of the quinine concentration. Based on the spectral deconvolution of the 1.00 g l(-1) solution, 52.4% +/- 0.7% and 52.7% +/- 0.7% of the signal in the visible range results from the quinine emission at 6 and 23 MV, respectively. The remaining fraction of the spectrum is due to the Cherenkov light that has not been converted. The fraction of the Cherenkov emission produced between 250 nm and 380 nm in the water and that was absorbed by the fluorophore reached 24.8% and 9.4% respectively at 6 and 23 MV. X-ray stimulated fluorescence of the quinine was then proven to be the principal cause to the increased total light output compared to the water-only signal. This new information reinforces the direct correlation of the solution intensity to the dose deposition.
Comparison of methods and achievable uncertainties for the relative and absolute measurement of photoluminescence quantum yields.[Pubmed:21473570]
Anal Chem. 2011 May 1;83(9):3431-9.
The photoluminescence quantum yield (Phi(f)) that presents a direct measure for the efficiency of the conversion of absorbed photons into emitted photons is one of the spectroscopic key parameters of functional fluorophores. It determines the suitability of such materials for applications in, for example, (bio)analysis, biosensing, and fluorescence imaging as well as as active components in optical devices. The reborn interest in accurate Phi(f) measurements in conjunction with the controversial reliability of reported Phi(f) values of many common organic dyes encouraged us to compare two relative and one absolute fluorometric method for the determination of the fluorescence quantum yields of Quinine sulfate dihydrate, coumarin 153, fluorescein, rhodamine 6G, and rhodamine 101. The relative methods include the use of a chain of Phi(f) transfer standards consisting of several "standard dye" versus "reference dye" pairs linked to a golden Phi(f) standard that covers the ultraviolet and visible spectral region, and the use of different excitation wavelengths for standard and sample, respectively. Based upon these measurements and the calibration of the instruments employed, complete uncertainty budgets for the resulting Phi(f) values are derived for each method, thereby providing evaluated standard operation procedures for Phi(f) measurements and, simultaneously, a set of assessed Phi(f) standards.
Evaluation of a commercial integrating sphere setup for the determination of absolute photoluminescence quantum yields of dilute dye solutions.[Pubmed:20615286]
Appl Spectrosc. 2010 Jul;64(7):733-41.
The commercial availability of stand-alone setups for the determination of absolute photoluminescence quantum yields (Phi(f)) in conjunction with the increasing use of integrating sphere accessories for spectrofluorometers is expected to have a considerable influence not only on the characterization of chromophore systems for use in optical and opto-electronic devices, but also on the determination of this key parameter for (bio)analytically relevant dyes and functional luminophores. Despite the huge potential of systems measuring absolute Phi(f) values and the renewed interest in dependable data, evaluated protocols for even the most elementary case, the determination of the fluorescence quantum yield of transparent dilute solutions of small organic dyes with integrating sphere methods, are still missing. This encouraged us to evaluate the performance and sources of uncertainty of a simple commercial integrating sphere setup with dilute solutions of two of the best characterized fluorescence quantum yield standards, Quinine sulfate dihydrate and rhodamine 101, strongly differing in spectral overlap between absorption and emission. Special attention is dedicated to illustrate common pitfalls of this approach, thereby deriving simple procedures to minimize measurement uncertainties and improve the comparability of data for the broad community of users of fluorescence techniques.
Redetermination of bis-{(1S,2S,4S,5R)-2-[(R)-hy-droxy(6-meth-oxy-4-quinol-yl)meth-yl]-5-vinyl-quinuc lidinium} sulfate dihydrate.[Pubmed:21588765]
Acta Crystallogr Sect E Struct Rep Online. 2010 Aug 28;66(Pt 9):o2443-4.
The structure of the title compound, known as Quinine sulfate dihydrate, 2C(20)H(25)N(2)O(2) (+).SO(4) (2-).2H(2)O, was previously reported by Mendel [Proc. K. Ned. Akad. Wet. (1955), 58, 132-134], but only the [010] projection was determined. Hence, we have redetermined its crystal structure at 100 K using three-dimensional data. The asymmetric unit consists of a quininium cation, viz. (R)-(6-meth-oxy-quinolinium-4-yl)[(1S,2S,4S,5R)-5-vinyl-quinuclid-in-ium-2-yl]met hanol, one half of a sulfate anion and a water mol-ecule. The S atom occupies a special position on a twofold axis. The packing is characterized by infinite columns, consisting of sulfate anions and water mol-ecules, linked through hydrogen bonds along the b axis, and further stabilized by hydrogen bonds to quininium cations. The quininium cations inter-act further through C-Hcdots, three dots, centeredO and C-Hcdots, three dots, centeredpi inter-actions.


