(S)-(-)-LimoneneCAS# 5989-54-8 |
- Limonene
Catalog No.:BCN3797
CAS No.:138-86-3
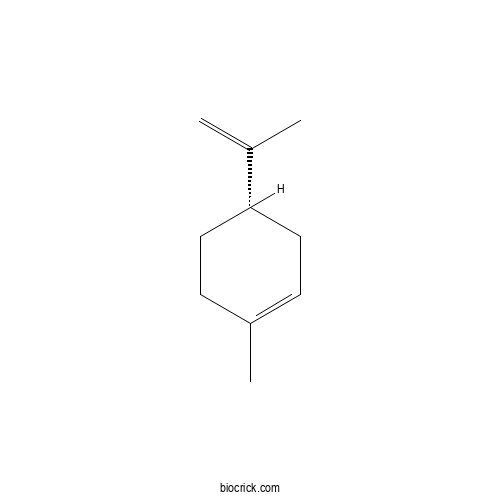
- (R)-(+)-Limonene
Catalog No.:BCN0067
CAS No.:5989-27-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5989-54-8 | SDF | Download SDF |
| PubChem ID | 439250 | Appearance | Oil |
| Formula | C10H16 | M.Wt | 136.2 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4S)-1-methyl-4-prop-1-en-2-ylcyclohexene | ||
| SMILES | CC1=CCC(CC1)C(=C)C | ||
| Standard InChIKey | XMGQYMWWDOXHJM-SNVBAGLBSA-N | ||
| Standard InChI | InChI=1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4,10H,1,5-7H2,2-3H3/t10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(S)-(-)-Limonene Dilution Calculator

(S)-(-)-Limonene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3421 mL | 36.7107 mL | 73.4214 mL | 146.8429 mL | 183.5536 mL |
| 5 mM | 1.4684 mL | 7.3421 mL | 14.6843 mL | 29.3686 mL | 36.7107 mL |
| 10 mM | 0.7342 mL | 3.6711 mL | 7.3421 mL | 14.6843 mL | 18.3554 mL |
| 50 mM | 0.1468 mL | 0.7342 mL | 1.4684 mL | 2.9369 mL | 3.6711 mL |
| 100 mM | 0.0734 mL | 0.3671 mL | 0.7342 mL | 1.4684 mL | 1.8355 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-(-)-Malic acid
Catalog No.:BCN9765
CAS No.:97-67-6
- Glucofrangulin B
Catalog No.:BCN9764
CAS No.:14062-59-0
- Dodeca 2E,4E,8Z,10E,Z-tetraenoic acid isobutylamide
Catalog No.:BCN9763
CAS No.:866602-52-0
- 2,3-Dihydro-2-phenyl-4H-benzopyran-4-one
Catalog No.:BCN9762
CAS No.:487-26-3
- Quinine sulfate dihydrate
Catalog No.:BCN9761
CAS No.:6119-70-6
- Rhoeadine
Catalog No.:BCN9760
CAS No.:2718-25-4
- Allylcysteine
Catalog No.:BCN9759
CAS No.:21593-77-1
- Ruberythric acid
Catalog No.:BCN9758
CAS No.:152-84-1
- Octanoic acid
Catalog No.:BCN9757
CAS No.:124-07-2
- 6,7-Dihydroxyflavone
Catalog No.:BCN9756
CAS No.:38183-04-9
- 5-Methoxyflavanone
Catalog No.:BCN9755
CAS No.:55947-36-9
- Arganine B
Catalog No.:BCN9754
CAS No.:144425-21-8
- Rebaudioside M
Catalog No.:BCN9767
CAS No.:1220616-44-3
- N-trans-caffeoyloctopamine
Catalog No.:BCN9768
CAS No.:1378868-10-0
- 2'-Methoxyflavone
Catalog No.:BCN9769
CAS No.:19725-47-4
- Comanthosid A
Catalog No.:BCN9770
CAS No.:70938-59-9
- (-)-Eburnamonine
Catalog No.:BCN9771
CAS No.:4880-88-0
- 4'-Methoxychalcone
Catalog No.:BCN9772
CAS No.:959-23-9
- DL-Tyrosine
Catalog No.:BCN9773
CAS No.:556-03-6
- Glucofrangulin A
Catalog No.:BCN9774
CAS No.:21133-53-9
- Nortrachelogenin-5'-C-beta-glucoside
Catalog No.:BCN9775
CAS No.:858127-39-6
- 3,5-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN9776
CAS No.:4319-02-2
- 3,4-Dimethoxyacetophenone
Catalog No.:BCN9777
CAS No.:1131-62-0
- Calvatic acid
Catalog No.:BCN9778
CAS No.:54723-08-9
Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids.[Pubmed:33268495]
Proc Natl Acad Sci U S A. 2020 Dec 2. pii: 2013968117.
Current approaches for the production of high-value compounds in microorganisms mostly use the cytosol as a general reaction vessel. However, competing pathways and metabolic cross-talk frequently prevent efficient synthesis of target compounds in the cytosol. Eukaryotic cells control the complexity of their metabolism by harnessing organelles to insulate biochemical pathways. Inspired by this concept, herein we transform yeast peroxisomes into microfactories for geranyl diphosphate-derived compounds, focusing on monoterpenoids, monoterpene indole alkaloids, and cannabinoids. We introduce a complete mevalonate pathway in the peroxisome to convert acetyl-CoA to several commercially important monoterpenes and achieve up to 125-fold increase over cytosolic production. Furthermore, peroxisomal production improves subsequent decoration by cytochrome P450s, supporting efficient conversion of (S)-(-)-Limonene to the menthol precursor trans-isopiperitenol. We also establish synthesis of 8-hydroxygeraniol, the precursor of monoterpene indole alkaloids, and cannabigerolic acid, the cannabinoid precursor. Our findings establish peroxisomal engineering as an efficient strategy for the production of isoprenoids.


