QuinizarinCAS# 81-64-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81-64-1 | SDF | Download SDF |
| PubChem ID | 6688 | Appearance | Powder |
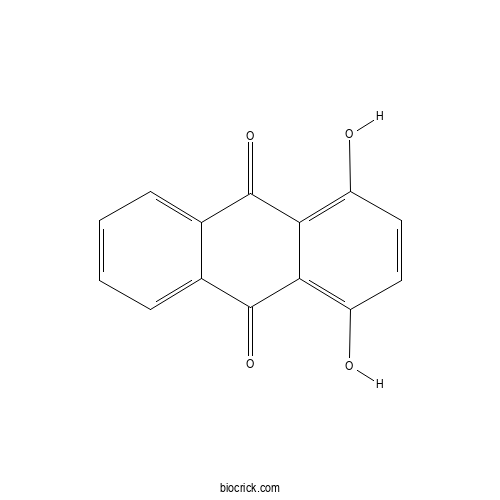
| Formula | C14H8O4 | M.Wt | 240.2 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,4-dihydroxyanthracene-9,10-dione | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C3=C(C=CC(=C3C2=O)O)O | ||
| Standard InChIKey | GUEIZVNYDFNHJU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H8O4/c15-9-5-6-10(16)12-11(9)13(17)7-3-1-2-4-8(7)14(12)18/h1-6,15-16H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Quinizarin exerts antiproliferative and antimetastatic activity on murine B16-F10 melanoma cells. It possesses significant antineoplastic properties, probably exerts through the induction of intracellular transglutaminase activity. Quinizarin exhibits a strong inhibition of Clostridium perfringens and moderate inhibition of Staphylococcus aureus without any adverse effects on the growth of Bifidobacterium adolescentis, B. bifidum, B. longum, and Lactobacillus casei. | |||||

Quinizarin Dilution Calculator

Quinizarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1632 mL | 20.816 mL | 41.632 mL | 83.2639 mL | 104.0799 mL |
| 5 mM | 0.8326 mL | 4.1632 mL | 8.3264 mL | 16.6528 mL | 20.816 mL |
| 10 mM | 0.4163 mL | 2.0816 mL | 4.1632 mL | 8.3264 mL | 10.408 mL |
| 50 mM | 0.0833 mL | 0.4163 mL | 0.8326 mL | 1.6653 mL | 2.0816 mL |
| 100 mM | 0.0416 mL | 0.2082 mL | 0.4163 mL | 0.8326 mL | 1.0408 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trioxsalen
Catalog No.:BCN9919
CAS No.:3902-71-4
- Geraldol
Catalog No.:BCN9918
CAS No.:21511-25-1
- DL-Malic acid
Catalog No.:BCN9917
CAS No.:617-48-1
- Crotonic acid
Catalog No.:BCN9916
CAS No.:107-93-7
- Tigogenin acetate
Catalog No.:BCN9915
CAS No.:2530-07-6
- (-)-Sparteine
Catalog No.:BCN9914
CAS No.:90-39-1
- Daucoidin A
Catalog No.:BCN9913
CAS No.:103629-87-4
- (R)-O-isobutyroyllomatin
Catalog No.:BCN9912
CAS No.:440094-38-2
- 5-Methoxypiperonal
Catalog No.:BCN9911
CAS No.:5780-07-4
- Corynanthine
Catalog No.:BCN9910
CAS No.:483-10-3
- Epoxybergamottin
Catalog No.:BCN9729
CAS No.:206978-14-5
- Calycopterin
Catalog No.:BCN9907
CAS No.:481-52-7
- 3'',4'',5,7-Tetrahydroxy 3,6,8-trimethoxyflavone
Catalog No.:BCN9921
CAS No.:61451-85-2
- (1S)-Chrysanthemolactone
Catalog No.:BCN9922
CAS No.:14087-71-9
- 2-(Beta-D-Glucopyranosyloxy)benzaldehyde
Catalog No.:BCN9923
CAS No.:618-65-5
- Demissidine
Catalog No.:BCN9924
CAS No.:474-08-8
- Furanoeudesma 1,3-diene
Catalog No.:BCN9925
CAS No.:87605-93-4
- trans-Stilbene
Catalog No.:BCN9926
CAS No.:103-30-0
- Sanguinarine nitrate
Catalog No.:BCN9927
CAS No.:4752-86-7
- 4-Phenylmorpholine
Catalog No.:BCN9928
CAS No.:92-53-5
- (+)-Dihydrocinchonine
Catalog No.:BCN9929
CAS No.:485-65-4
- 6-Methoxyflavone
Catalog No.:BCN9930
CAS No.:26964-24-9
- Pseudopelletierine hydrochloride
Catalog No.:BCN9931
CAS No.:6164-62-1
- 3-Carene
Catalog No.:BCN9932
CAS No.:13466-78-9
Biophysical Characterization and Anticancer Activities of Photosensitive Phytoanthraquinones Represented by Hypericin and Its Model Compounds.[Pubmed:33271809]
Molecules. 2020 Dec 1;25(23). pii: molecules25235666.
Photosensitive compounds found in herbs have been reported in recent years as having a variety of interesting medicinal and biological activities. In this review, we focus on photosensitizers such as hypericin and its model compounds emodin, Quinizarin, and danthron, which have antiviral, antifungal, antineoplastic, and antitumor effects. They can be utilized as potential agents in photodynamic therapy, especially in photodynamic therapy (PDT) for cancer. We aimed to give a comprehensive summary of the physical and chemical properties of these interesting molecules, emphasizing their mechanism of action in relation to their different interactions with biomacromolecules, specifically with DNA.
Antiproliferative, DNA binding, and cleavage properties of dinuclear Co(III) complexes containing the bioactive quinizarin ligand.[Pubmed:32112290]
J Biol Inorg Chem. 2020 Mar;25(2):339-350.
The adverse side effects and acquired resistance associated with the clinical application of traditional platinum-based anticancer drugs have forced investigation of alternative transition metal-based compounds and their cytostatic properties. Over the last years, the anticancer potential of cobalt complexes has been extensively studied, and in-depth analyses of their mode of action have been conducted. In this work, we present antiproliferative activity against human cancer cells of the dinuclear Co(III) complexes bearing the Quinizarin ligand and tris(2-aminoethyl)amine (tren, compound 1) or tris(2-pyridylmethyl)amine (tpa, compound 2) co-ligands. To contribute the understanding mechanisms of biological action of these compounds, their association with DNA in the cells, DNA binding in cell-free media, and DNA cleavage capability were investigated in detail. The results demonstrate that both complexes interact with DNA in tumor cells. However, their mechanism of antiproliferative action is different, and this difference is mirrored by distinct antiproliferative activity. The antiproliferative effect of 1 is connected with its ability to intercalate into DNA and subsequently to inhibit activities of DNA processing enzymes. In contrast, the total antiproliferative efficiency of 2, thanks to its redox properties, appears to be connected with its ability to form radicals and, consequently, with the ability of 2 to cleave DNA. Hence, the findings presented in this study may significantly contribute to understanding the antitumor potential of cobalt complexes. Dinuclear Co(III) complexes containing the bioactive Quinizarin ligand exhibit antiproliferative activity based on distinct mechanism.
Biosynthesis of Rhamnosylated Anthraquinones in Escherichia coli.[Pubmed:31893599]
J Microbiol Biotechnol. 2020 Mar 28;30(3):398-403.
Rhamnose is a naturally occurring deoxysugar present as a glycogenic component of plant and microbial natural products. A recombinant mutant Escherichia coli strain was developed by overexpressing genes involved in the TDP-L-rhamnose biosynthesis pathway of different bacterial strains and Saccharothrix espanaensis rhamnosyl transferase to conjugate intrinsic cytosolic TDP-L-rhamnose with anthraquinones supplemented exogenously. Among the five anthraquinones (alizarin, emodin, chrysazin, anthrarufin, and Quinizarin) tested, Quinizarin was biotransformed into a rhamoside derivative with the highest conversion ratio by whole cells of engineered E. coli. The Quinizarin glycoside was identified by various chromatographic and spectroscopic analyses. The anti-proliferative property of the newly synthesized rhamnoside, Quinizarin-4-O-alpha-L-rhamnoside, was assayed in various cancer cells.
Synthesis, characterization and albumin binding capabilities of quinizarin containing ternary cobalt(III) complexes.[Pubmed:31874363]
J Inorg Biochem. 2020 Mar;204:110963.
Four Co(III) ternary complexes with the composition of [(Co(4 N))2(quin)](ClO4)4 or [(Co(4 N))2(quinS)](ClO4)3, where 4 N = tris(2-aminoethyl)amine (tren) or tris(2-pyridylmethyl)amine (tpa), quinH2 = Quinizarin (1,4-dihydroxy-9,10-anthraquinone), quinSH3 = Quinizarin-2-sulfonic acid (1,4-dihydroxy-9,10-anthraquinone-2-sulfonic acid), were synthesized, characterized and their human serum albumin (HSA) binding capabilities were also tested. The complexes can be considered as likely chaperons of Quinizarins which are structural models for anthracycline-based anticancer drugs like doxorubicin. All the Co(III) complexes are dinuclear and were isolated as mixture of isomers. Comparison of the cyclic voltammograms of the free ligands and the appropriate Co(III) complexes revealed that the new signals belonging to reversible processes in the range -400-0 mV (vs. Ag/AgCl) for the complexes can be attributed to the reversible reduction of the Co(III) centre. These potentials are in the range of typical (O,O) chelated Co(III) ternary complexes bearing 4 N donor ligands and follow the order being more positive for the tpa containing complexes. Presence of the sulfonate group in the Quinizarin results in slightly more negative reduction potential of the Co(III) complexes. HSA binding capabilities of the quinH2 and quinSH3 ligands as well as the appropriate complexes showed that quinSH3 has higher affinity to the protein than quinH2 while none of the complexes seem to bind to HSA.
Powder and Nanotubes Titania Modified by Dye Sensitization as Photocatalysts for the Organic Pollutants Elimination.[Pubmed:30987003]
Nanomaterials (Basel). 2019 Apr 2;9(4). pii: nano9040517.
In this study, titanium dioxide powder obtained by the sol-gel method and TiO(2) nanotubes, were prepared. In order to increase the TiO(2) photoactivity, the powders and nanotubes obtained were modified by dye sensitization treatment during the oxide synthesis. The sensitizers applied were Quinizarin (Q) and Zinc protoporphyrin (P). The materials synthesized were extensively characterized and it was found that the dye sensitization treatment leads to modify the optical and surface properties of Titania. It was also found that the effectiveness of the dye-sensitized catalysts in the phenol and methyl orange (MO) photodegradation strongly depends on the dye sensitizer employed. Thus, the highest degradation rate for MO was obtained over the conventional Q-TiO(2) photocatalyst. In the case of the nanotubes series, the most effective photocatalyst in the MO degradation was based on TiO(2)-nanotubes sensitized with the dye protoporfirin (ZnP). Selected catalysts were also tested in the phenol and MO photodegradation under visible light and it was observed that these samples are also active under this radiation.
Design and synthesis of various quinizarin derivatives as potential anticancer agents in acute T lymphoblastic leukemia.[Pubmed:30827866]
Bioorg Med Chem. 2019 Apr 1;27(7):1362-1369.
A series of Quinizarin derivatives containing quaternary ammonium salts and/or thiourea groups were synthesized and their anticancer activities against leukemia cell lines have been tested. Results showed that most of Quinizarin derivatives could inhibit the proliferation of leukemia cells. Among these derivatives, compound 3 showed good inhibition activity against various leukemia cells with IC50 values ranging from 0.90+/-2.55muM to 10.90+/-3.66muM. At the same time, compound 3 also inhibited the growth of human embryonic kidney-293 cell (HEK-293). Molt-4 and Jurkat cells, acute T lymphoblastic leukemia (T-ALL) cell lines, were selected to reveal potential anticancer mechanism of compound 3. Compound 3 inhibited the proliferation of Molt-4 and Jurkat cells in a dose- and time-dependent manner and led to a marked G0/G1 phase arrest. Analysis of Annexin V-APC and intracellular reactive oxygen species (ROS) level by flow cytometry showed that compound 3 induced significant apoptosis in Molt-4 and Jurkat cells. Western blotting assay showed that compound 3 activated the caspase-dependent apoptosis pathway and induced the degradation of Bcl-2 and c-myc protein.
Building Molecular Complexity from Quinizarin: Conjoined Coumarins and Coronene Analogs.[Pubmed:30022613]
Chem Asian J. 2019 May 15;14(10):1763-1770.
The double Knoevenagel condensation of 1,4-dibenzoyloxyanthraquinone with methyl esters of arylacetic acids affords a series of compounds based upon a previously unknown 1,8-dioxa-benzo[e]pyrene-2,7-dione heterocyclic core. The aryl groups incorporated in the 3- and 6-positions can be oxidatively coupled to the pi-expanded backbone to produce a further new heterocyclic core: 1,10-dioxa-dibenzo[dj]coronene-2,9-dione. The intriguing optical properties of these pi-expanded coumarin derivatives are discussed and rationalized through quantum chemical calculations. The broad absorption bands of 1,8-dioxa-benzo[e]pyrene-2,7-dione-based dyes are attributed to both HOMO-1-->LUMO and HOMO-->LUMO transitions, which have a similar energy. Weakly coupled electron-donating aryl substituents result in a moderate bathochromic shift of both the absorption and emission by 30-60 nm in toluene. The emissive properties of these compounds are in part determined by the oscillator strength of the main transition, lifetimes of the excited state, and by the energy match of the excited state with a triplet state of a similar energy. The 1,10-dioxa-dibenzo[dj]coronene-2,9-dione displays a much smaller Stokes shift, yet a markedly increased fluorescence quantum yield of 90 % owing to the increased rigidity compared with the 1,8-dioxa-benzo[e]pyrene-2,7-dione core.
Soret forced Rayleigh scattering instrument for simultaneous detection of two-wavelength signals to measure Soret coefficient and thermodiffusion coefficient in ternary mixtures.[Pubmed:29495814]
Rev Sci Instrum. 2018 Feb;89(2):024903.
We describe an instrument for the measurement of the Soret and thermodiffusion coefficients in ternary systems based on the transient holographic grating technique, which is called Soret forced Rayleigh scattering (SFRS) or thermal diffusion forced Rayleigh scattering (TDFRS). We integrated the SFRS technique and the two-wavelength detection technique, which enabled us to obtain two different signals to determine the two independent Soret coefficients and thermodiffusion coefficients in ternary systems. The instrument has been designed to read the mass transport simultaneously by two-wavelength lasers with wavelengths of lambda = 403 nm and lambda = 639 nm. The irradiation time of the probing lasers is controlled to reduce the effect of laser absorption to the sample with dye (Quinizarin), which is added to convert the interference pattern of the heating laser of lambda = 532 nm to the temperature grating. The result of the measurement of binary benchmark mixtures composed of 1,2,3,4-tetrahydronaphthalene (THN), isobutylbenzene (IBB), and n-dodecane (nC12) shows that the simultaneous two-wavelength observation of the Soret effect and the mass diffusion are adequately performed. To evaluate performance in the measurement of ternary systems, we carried out experiments on the ternary benchmark mixtures of THN/IBB/nC12 with the mass fractions of 0.800/0.100/0.100 at a temperature of 298.2 K. The Soret coefficient and thermodiffusion coefficient agreed with the ternary benchmark values within the range of the standard uncertainties (23% for the Soret coefficient of THN and 30% for the thermodiffusion coefficient of THN).
Crystal structures of 1-hy-droxy-4-prop-yloxy-9,10-anthra-quinone and its acetyl derivative.[Pubmed:29250400]
Acta Crystallogr E Crystallogr Commun. 2017 Nov 10;73(Pt 12):1845-1849.
1-Hy-droxy-4-prop-yloxy-9,10-anthra-quinone, C17H14O4, (I), and its acetyl derivative, 4-acet-yloxy-4-prop-yloxy-9,10-anthra-quinone, C19H16O5, (II), were synthesized from the commercially available dye Quinizarin. In both compounds, the anthra-quinone frameworks are close to planarity. There is a large difference in the conformation of the prop-yloxy group; the mol-ecule of (I) adopts a gauche conformation [O-C-C-C = -64.4 (2) degrees ], although the mol-ecule of (II) takes a trans-planar conformation (zigzag) [O-C-C-C = 176.1 (3) degrees ]. In the mol-ecule of (I), there is an intra-molecular O-Hcdots, three dots, centeredO hydrogen bond. In both crystals, the mol-ecules are linked by C-H cdots, three dots, centeredO hydrogen bonds. A difference in the mol-ecular arrangements of (I) and (II) is found along the stacking directions.
Monitoring the Activity of Immobilized Lipase with Quinizarin Diester Fluoro-Chromogenic Probe.[Pubmed:29207517]
Molecules. 2017 Dec 4;22(12). pii: molecules22122136.
Quinizarin diester is used as a fluoro-chromogenic substrate of the activity of lipase supported in poly(methylmetacrylate) beads (CALB, Novozym((R)) 435) dispersed in organic solvents. The monoester and diester of Quinizarin are both non-fluorescent species contrasting with the enzymatic product Quinizarin that shows optical absorption in the visible region and strong fluorescence signal. The enzymatic conversion is accomplished by spectroscopic measurements and it follows a sigmoid curve from which the mean reaction time of the enzymatic process can be determined. This parameter indicates the enzyme activity of the immobilized lipase. Its dependency with the amount of lipase allowed the determination of the ratio of the catalytic rate and the Michaelis constant (kc/Km) and the experimental value found was (1.0 +/- 0.1) x 10(-2) mg(-1)/min in the case of Quinizarin diacetate.


