RunanineCAS# 100485-12-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100485-12-9 | SDF | Download SDF |
| PubChem ID | 119026174 | Appearance | Powder |
| Formula | C21H27NO5 | M.Wt | 373.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
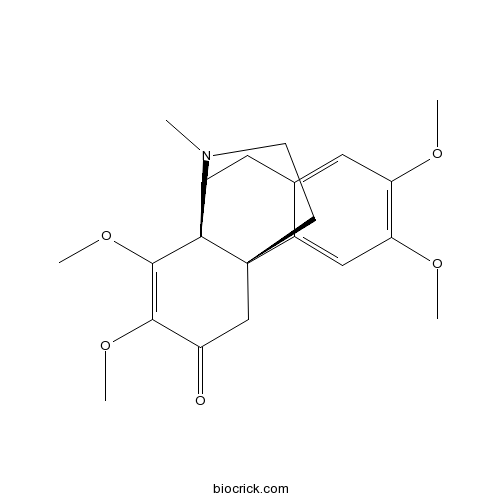
| Chemical Name | (1S,10S)-4,5,11,12-tetramethoxy-17-methyl-17-azatetracyclo[8.4.3.01,10.02,7]heptadeca-2,4,6,11-tetraen-13-one | ||
| SMILES | CN1CCC23C1(CCC4=CC(=C(C=C42)OC)OC)C(=C(C(=O)C3)OC)OC | ||
| Standard InChIKey | FFKKIUDOINNTGR-LEWJYISDSA-N | ||
| Standard InChI | InChI=1S/C21H27NO5/c1-22-9-8-20-12-15(23)18(26-4)19(27-5)21(20,22)7-6-13-10-16(24-2)17(25-3)11-14(13)20/h10-11H,6-9,12H2,1-5H3/t20-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Runanine Dilution Calculator

Runanine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6781 mL | 13.3905 mL | 26.7809 mL | 53.5619 mL | 66.9523 mL |
| 5 mM | 0.5356 mL | 2.6781 mL | 5.3562 mL | 10.7124 mL | 13.3905 mL |
| 10 mM | 0.2678 mL | 1.339 mL | 2.6781 mL | 5.3562 mL | 6.6952 mL |
| 50 mM | 0.0536 mL | 0.2678 mL | 0.5356 mL | 1.0712 mL | 1.339 mL |
| 100 mM | 0.0268 mL | 0.1339 mL | 0.2678 mL | 0.5356 mL | 0.6695 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Isoampelopsin F
Catalog No.:BCN9585
CAS No.:354553-38-1
- 3'-Hydroxy-3,5,6,7,8,4',5'-heptamethoxyflavone
Catalog No.:BCN9584
CAS No.:5244-28-0
- Goyaglycoside d
Catalog No.:BCN9583
CAS No.:333332-50-6
- Actinodaphnine
Catalog No.:BCN9582
CAS No.:517-69-1
- Fortunolide A
Catalog No.:BCN9581
CAS No.:252574-51-9
- Hainanolidol
Catalog No.:BCN9580
CAS No.:73213-63-5
- Karaviloside XI
Catalog No.:BCN9579
CAS No.:934739-35-2
- Taikuguasin D
Catalog No.:BCN9578
CAS No.:1627163-80-7
- Momordicin IV
Catalog No.:BCN9577
CAS No.:894412-35-2
- Demethylagrimonolide 6-O-glucoside
Catalog No.:BCN9576
CAS No.:1257408-55-1
- Caryatin
Catalog No.:BCN9575
CAS No.:1486-66-4
- Harringtonolide
Catalog No.:BCN9574
CAS No.:64761-48-4
- Blumenol B 9-O-glucoside
Catalog No.:BCN9587
CAS No.:114226-08-3
- Isostephodeline
Catalog No.:BCN9588
CAS No.:56648-85-2
- 11β,13-Dihydrotaraxinic acid
Catalog No.:BCN9589
CAS No.:1274668-83-5
- 3'-Hydroxy-3,5,8,4',5'-pentamethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN9590
CAS No.:82668-94-8
- Taccaoside E
Catalog No.:BCN9591
CAS No.:1858199-00-4
- Boschnaloside
Catalog No.:BCN9592
CAS No.:72963-55-4
- 6a,7-Dehydroboldine
Catalog No.:BCN9593
CAS No.:91599-23-4
- Citrusinine I
Catalog No.:BCN9594
CAS No.:86680-32-2
- Junosine
Catalog No.:BCN9595
CAS No.:103956-34-9
- Tannagine
Catalog No.:BCN9596
CAS No.:123750-34-5
- Sinococuline
Catalog No.:BCN9597
CAS No.:109351-36-2
- (2R,3R)-Glucodistylin
Catalog No.:BCN9598
CAS No.:27297-45-6
Memory of Chirality in Bromoalkyne Carbocyclization: Applications in Asymmetric Total Synthesis of Hasubanan Alkaloids.[Pubmed:30548073]
Org Lett. 2019 Jan 4;21(1):292-295.
A transition-metal-free 5- exo- dig asymmetric cyclization of alpha-amino ester enolates onto bromoalkynes provided a product with excellent enantioselectivity via the memory of chirality concept. This strategy was applied to a concise total synthesis of (-)-Runanine and a formal synthesis of (-)-8-demethoxyRunanine and (-)-cepharatine D.
The Hasubanan and Acutumine Alkaloids.[Pubmed:26521650]
Alkaloids Chem Biol. 2014;73:161-222.
Research in the hasubanan and acutumine alkaloid fields up to 1970 was discussed under "morphine alkaloids" in Volume 13 of this chapter. Advances in the field of hasubanan alkaloids from 1971 to 1975 were reviewed in Volume 16 and from 1976 to 1986 in Volume 33. This chapter extends the information in the three preceding reviews to hasubanan alkaloid literature published from 1987 to June 2013. This chapter covers acutumine alkaloid literature since (-)-acutumine (3) was isolated in 1929. This chapter includes occurrence and physical constants, new alkaloids, synthesis, biosynthesis, and pharmacology. Section 1 introduces the foremost alkaloids, (-)-hasubanonine (1) and (-)-acutumine (3), and the numbering systems of the hasubanan (2) and acutumine (4) skeletons. Section 2 details the occurrence and physical constants of 29 new hasubanan and 15 acutumine alkaloids. The isolation and structural determination of these new alkaloids are described in Section 3. Section 4 summarizes total syntheses and synthetic studies toward hasubanan and acutumine alkaloids. Completed syntheses of the hasubanan alkaloids (+)-cepharamine (ent-71), (-)-hasubanonine (1), (-)-Runanine (8), (-)-delavayine (6), (+)-periglaucine B (19), and (-)-8-demethoxyRunanine (12) are reviewed. Completed syntheses of (-)-acutumine (3) and (-)-dechloroacutumine (52) are also described. Section 5 details biosyntheses of (-)-acutumine (3) advanced by Barton, Wipf, and Sugimoto. Section 6 summarizes pharmacological studies of hasubanan and acutumine alkaloids. Opioid receptor affinity, anti-HBV activity, and antimicrobial activity of hasubanan alkaloids are reported. Antiamnesic properties, cytotoxicity, and anti-HBV activity of acutumine alkaloids are described.
Synthesis of isohasubanan alkaloids via enantioselective ketone allylation and discovery of an unexpected rearrangement.[Pubmed:19072324]
J Org Chem. 2009 Feb 6;74(3):1187-99.
A synthesis of the hasubanan alkaloids hasubanonine, Runanine, and aknadinine via a unified route was attempted. Construction of key phenanthrene intermediates by a Suzuki coupling-Wittig olefination-ring-closing metathesis sequence allowed a convergent and flexible approach. Conversion of the phenanthrenes into the target structures was projected to involve six steps including phenolic oxidation, ketone allylation, anionic oxy-Cope rearrangement, and acid-promoted cyclization. The final step was thwarted by a pinacol-like rearrangement that delivered the unnatural isohasubanan alkaloid skeleton. The structures of the products were established by exhaustive NMR experiments and confirmed by GIAO (13)C NMR calculations of Runanine, isoRunanine, and three other isomers. These computations revealed some inconsistencies with the benzene solvent correction which suggest that caution should be used in employing this algorithm. The racemic synthesis of isohasubanonine was transformed into an enantioselective synthesis by the discovery that Nakamura's chiral bisoxazoline-ligated allylzinc reagent mediates the enantioselective allylation of ketone 19 in 93% ee. This method could be extended to three other structurally related ketones (92-96% ee), and the enantioselective syntheses of two other isohasubanan alkaloids, isoRunanine and isoaknadinine, were accomplished. Racemic isohasubanonine was found to be an ineffective analgesic agent.


