CaryatinCAS# 1486-66-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1486-66-4 | SDF | Download SDF |
| PubChem ID | 5489501 | Appearance | Yellow powder |
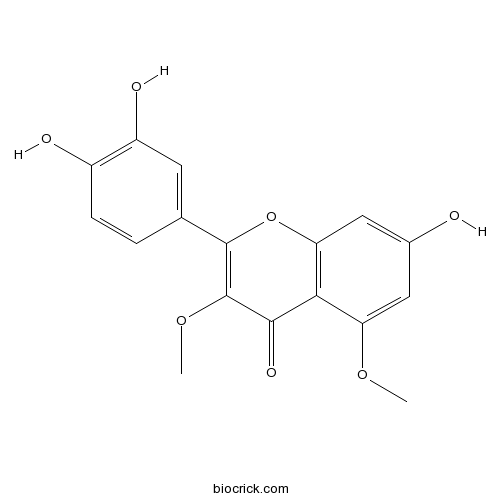
| Formula | C17H14O7 | M.Wt | 330.29 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)-7-hydroxy-3,5-dimethoxychromen-4-one | ||
| SMILES | COC1=CC(=CC2=C1C(=O)C(=C(O2)C3=CC(=C(C=C3)O)O)OC)O | ||
| Standard InChIKey | AOFQCVDYMNHCKD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O7/c1-22-12-6-9(18)7-13-14(12)15(21)17(23-2)16(24-13)8-3-4-10(19)11(20)5-8/h3-7,18-20H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Caryatin Dilution Calculator

Caryatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0276 mL | 15.1382 mL | 30.2764 mL | 60.5528 mL | 75.6911 mL |
| 5 mM | 0.6055 mL | 3.0276 mL | 6.0553 mL | 12.1106 mL | 15.1382 mL |
| 10 mM | 0.3028 mL | 1.5138 mL | 3.0276 mL | 6.0553 mL | 7.5691 mL |
| 50 mM | 0.0606 mL | 0.3028 mL | 0.6055 mL | 1.2111 mL | 1.5138 mL |
| 100 mM | 0.0303 mL | 0.1514 mL | 0.3028 mL | 0.6055 mL | 0.7569 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Harringtonolide
Catalog No.:BCN9574
CAS No.:64761-48-4
- Taraxinic acid
Catalog No.:BCN9573
CAS No.:75911-33-0
- 1,7-Bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one
Catalog No.:BCN9572
CAS No.:149732-52-5
- Isohopeaphenol
Catalog No.:BCN9571
CAS No.:197446-77-8
- Hopeaphenol
Catalog No.:BCN9570
CAS No.:388582-37-4
- Myricetin 3,7,3'-trimethyl ether 5'-O-glucoside
Catalog No.:BCN9569
CAS No.:2170444-56-9
- 3,4-Dihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9568
CAS No.:454473-97-3
- 3,4,5-Trihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9567
CAS No.:2172431-63-7
- Pukateine
Catalog No.:BCN9566
CAS No.:81-67-4
- 11-Methylforsythide
Catalog No.:BCN9565
CAS No.:159598-00-2
- Adoxoside
Catalog No.:BCN9564
CAS No.:42830-26-2
- 3-O-Methylellagic acid 4-O-rhamnoside
Catalog No.:BCN9563
CAS No.:639089-97-7
- Demethylagrimonolide 6-O-glucoside
Catalog No.:BCN9576
CAS No.:1257408-55-1
- Momordicin IV
Catalog No.:BCN9577
CAS No.:894412-35-2
- Taikuguasin D
Catalog No.:BCN9578
CAS No.:1627163-80-7
- Karaviloside XI
Catalog No.:BCN9579
CAS No.:934739-35-2
- Hainanolidol
Catalog No.:BCN9580
CAS No.:73213-63-5
- Fortunolide A
Catalog No.:BCN9581
CAS No.:252574-51-9
- Actinodaphnine
Catalog No.:BCN9582
CAS No.:517-69-1
- Goyaglycoside d
Catalog No.:BCN9583
CAS No.:333332-50-6
- 3'-Hydroxy-3,5,6,7,8,4',5'-heptamethoxyflavone
Catalog No.:BCN9584
CAS No.:5244-28-0
- (+)-Isoampelopsin F
Catalog No.:BCN9585
CAS No.:354553-38-1
- Runanine
Catalog No.:BCN9586
CAS No.:100485-12-9
- Blumenol B 9-O-glucoside
Catalog No.:BCN9587
CAS No.:114226-08-3
Antitumor-promoting constituents from Dioscorea bulbifera L. in JB6 mouse epidermal cells.[Pubmed:12230129]
Biol Pharm Bull. 2002 Sep;25(9):1241-3.
An antitumor-promoting effect was found in the extracts/ingredients of a plant used as a traditional medicine in mainland China, using the neoplastic transformation assay of mouse epidermal JB6 cell lines. The ethyl acetate soluble fraction of 75% ethanol extract of the rhizomes of Dioscorea bulbifera L. showed an inhibitory effect against the tumor promotion of JB6 (Cl 22 and Cl 41) cells induced by a promoter, 12-O-tetradecanoylphorbol-13-acetate (TPA). Further investigation on the constituents of the EtOAc fraction from the rhizomes revealed the chemical structure to be kaempferol-3,5-dimethyl ether (1), Caryatin (2), (+)-catechin (3), myricetin (4), quercetin-3-O-galactopyranoside (5), myricetin-3-O-galactopyranoside (6), myricetin-3-O-glucopyranoside (7) and diosbulbin B (8). Constituent antitumor-promoting activities were also examined in the same way. Compounds 1-7, characterized as flavonoids with the two hydroxyl groups at C-7 and C-4', showed the most potent inhibitory effect, but there seemed to be differences in the inhibitory effect between flavonol aglycones and flavonol glycosides. Compared with (-)-epicatechin, (+)-catechin exhibited much stronger inhibitory activity which suggested that chemical stereo structures of compounds affect the efficiency of inhibition. Compound 8 showed moderate activity. The constituents with antitumor-promoting activity from this plant are reported for the first time.


