HopeaphenolCAS# 388582-37-4 |
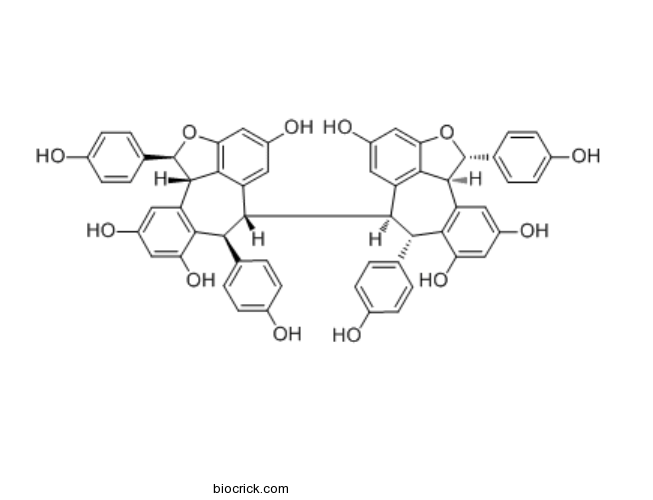
- Isohopeaphenol
Catalog No.:BCN9571
CAS No.:197446-77-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 388582-37-4 | SDF | Download SDF |
| PubChem ID | 21669381 | Appearance | Brown powder |
| Formula | C56H42O12 | M.Wt | 906.9 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,8S,9R,16S)-8,16-bis(4-hydroxyphenyl)-9-[(1S,8S,9R,16S)-4,6,12-trihydroxy-8,16-bis(4-hydroxyphenyl)-15-oxatetracyclo[8.6.1.02,7.014,17]heptadeca-2(7),3,5,10(17),11,13-hexaen-9-yl]-15-oxatetracyclo[8.6.1.02,7.014,17]heptadeca-2(7),3,5,10(17),11,13-hexaene-4,6,12-triol | ||
| SMILES | C1=CC(=CC=C1C2C(C3=C4C(C(OC4=CC(=C3)O)C5=CC=C(C=C5)O)C6=C2C(=CC(=C6)O)O)C7C(C8=C(C=C(C=C8O)O)C9C(OC1=CC(=CC7=C91)O)C1=CC=C(C=C1)O)C1=CC=C(C=C1)O)O | ||
| Standard InChIKey | YQQUILZPDYJDQJ-QWJFNKQSSA-N | ||
| Standard InChI | InChI=1S/C56H42O12/c57-29-9-1-25(2-10-29)45-47-37(17-33(61)21-41(47)65)53-49-39(19-35(63)23-43(49)67-55(53)27-5-13-31(59)14-6-27)51(45)52-40-20-36(64)24-44-50(40)54(56(68-44)28-7-15-32(60)16-8-28)38-18-34(62)22-42(66)48(38)46(52)26-3-11-30(58)12-4-26/h1-24,45-46,51-66H/t45-,46-,51-,52-,53-,54-,55+,56+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hopeaphenol Dilution Calculator

Hopeaphenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1027 mL | 5.5133 mL | 11.0266 mL | 22.0531 mL | 27.5664 mL |
| 5 mM | 0.2205 mL | 1.1027 mL | 2.2053 mL | 4.4106 mL | 5.5133 mL |
| 10 mM | 0.1103 mL | 0.5513 mL | 1.1027 mL | 2.2053 mL | 2.7566 mL |
| 50 mM | 0.0221 mL | 0.1103 mL | 0.2205 mL | 0.4411 mL | 0.5513 mL |
| 100 mM | 0.011 mL | 0.0551 mL | 0.1103 mL | 0.2205 mL | 0.2757 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Myricetin 3,7,3'-trimethyl ether 5'-O-glucoside
Catalog No.:BCN9569
CAS No.:2170444-56-9
- 3,4-Dihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9568
CAS No.:454473-97-3
- 3,4,5-Trihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9567
CAS No.:2172431-63-7
- Pukateine
Catalog No.:BCN9566
CAS No.:81-67-4
- 11-Methylforsythide
Catalog No.:BCN9565
CAS No.:159598-00-2
- Adoxoside
Catalog No.:BCN9564
CAS No.:42830-26-2
- 3-O-Methylellagic acid 4-O-rhamnoside
Catalog No.:BCN9563
CAS No.:639089-97-7
- Quercetin 3,5,3'-trimethyl ether
Catalog No.:BCN9562
CAS No.:13459-09-1
- Aesculioside C
Catalog No.:BCN9561
CAS No.:254896-65-6
- Isoaesculioside D
Catalog No.:BCN9560
CAS No.:1184581-59-6
- Sonderianol
Catalog No.:BCN9559
CAS No.:85563-65-1
- Vanicoside B
Catalog No.:BCN9558
CAS No.:155179-21-8
- Isohopeaphenol
Catalog No.:BCN9571
CAS No.:197446-77-8
- 1,7-Bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one
Catalog No.:BCN9572
CAS No.:149732-52-5
- Taraxinic acid
Catalog No.:BCN9573
CAS No.:75911-33-0
- Harringtonolide
Catalog No.:BCN9574
CAS No.:64761-48-4
- Caryatin
Catalog No.:BCN9575
CAS No.:1486-66-4
- Demethylagrimonolide 6-O-glucoside
Catalog No.:BCN9576
CAS No.:1257408-55-1
- Momordicin IV
Catalog No.:BCN9577
CAS No.:894412-35-2
- Taikuguasin D
Catalog No.:BCN9578
CAS No.:1627163-80-7
- Karaviloside XI
Catalog No.:BCN9579
CAS No.:934739-35-2
- Hainanolidol
Catalog No.:BCN9580
CAS No.:73213-63-5
- Fortunolide A
Catalog No.:BCN9581
CAS No.:252574-51-9
- Actinodaphnine
Catalog No.:BCN9582
CAS No.:517-69-1
Wood Metabolomic Responses of Wild and Cultivated Grapevine to Infection with Neofusicoccum parvum, a Trunk Disease Pathogen.[Pubmed:32512855]
Metabolites. 2020 Jun 4;10(6). pii: metabo10060232.
Grapevine trunk diseases (GTDs), which are associated with complex of xylem-inhabiting fungi, represent one of the major threats to vineyard sustainability currently. Botryosphaeria dieback, one of the major GTDs, is associated with wood colonization by Botryosphaeriaceae fungi, especially Neofusicoccum parvum. We used GC-MS and HPLC-MS to compare the wood metabolomic responses of the susceptible Vitis vinifera subsp. vinifera (V.v. subsp. vinifera) and the tolerant Vitis vinifera subsp. sylvestris (V.v. subsp. sylvestris) after artificial inoculation with Neofusicoccum parvum (N. parvum). N. parvum inoculation triggered major changes in both primary and specialized metabolites in the wood. In both subspecies, infection resulted in a strong decrease in sugars (fructose, glucose, sucrose), whereas sugar alcohol content (mannitol and arabitol) was enhanced. Concerning amino acids, N. parvum early infection triggered a decrease in aspartic acid, serine, and asparagine, and a strong increase in alanine and -alanine. A trend for more intense primary metabolism alteration was observed in V.v. subsp. sylvestris compared to V. v. subsp. vinifera. N. parvum infection also triggered major changes in stilbene and flavonoid compounds. The content in resveratrol and several resveratrol oligomers increased in the wood of both subspecies after infection. Interestingly, we found a higher induction of resveratrol oligomer (putative E-miyabenol C, vitisin C, Hopeaphenol, ampelopsin C) contents after wood inoculation in V.v. subsp. sylvestris.
Screening of Natural Stilbene Oligomers from Vitis vinifera for Anticancer Activity on Human Hepatocellular Carcinoma Cells.[Pubmed:32492881]
Antioxidants (Basel). 2020 Jun 1;9(6). pii: antiox9060469.
The characterization of bioactive resveratrol oligomers extracted from Vitis vinifera canes has been recently reported. Here, we screened six of these compounds (ampelopsin A, trans-epsilon-viniferin, Hopeaphenol, isoHopeaphenol, R2-viniferin, and R-viniferin) for their cytotoxic activity to human hepatocellular carcinoma (HCC) cell lines p53 wild-type HepG2 and p53-null Hep3B. The cytotoxic efficacy depended on the cell line. R2-viniferin was the most toxic stilbene in HepG2, with inhibitory concentration 50 (IC50) of 9.7 +/- 0.4 microM at 72 h, 3-fold lower than for resveratrol, while Hep3B was less sensitive (IC50 of 47.8 +/- 2.8 microM). By contrast, Hopeaphenol (IC50 of 13.1 +/- 4.1 microM) and isoHopeaphenol (IC50 of 26.0 +/- 3.0 microM) were more toxic to Hep3B. Due to these results, and because it did not exert a large cytotoxicity in HH4 non-transformed hepatocytes, R2-viniferin was selected to investigate its mechanism of action in HepG2. The stilbene tended to arrest cell cycle at G2/M, and it also increased intracellular reactive oxygen species (ROS), caspase 3 activity, and the ratio of Bax/Bcl-2 proteins, indicative of apoptosis. The distinctive toxicity of R2-viniferin on HepG2 encourages research into the underlying mechanism to develop the oligostilbene as a therapeutic agent against HCC with a particular genetic background.
Grape Cane Extracts as Multifunctional Rejuvenating Cosmetic Ingredient: Evaluation of Sirtuin Activity, Tyrosinase Inhibition and Bioavailability Potential.[Pubmed:32397228]
Molecules. 2020 May 8;25(9). pii: molecules25092203.
Grape canes are waste biomass of viticulture containing bioactive polyphenols valuable in cosmetics. Whereas several studies reported the cosmetic activities of E-resveratrol, only few described the potential of E-epsilon-viniferin, the second major constituent of grape cane extracts (GCE), and none of them investigated GCE as a natural blend of polyphenols for cosmetic applications. In this study, we considered the potential of GCE from polyphenol-rich grape varieties as multifunctional cosmetic ingredients. HPLC analysis was performed to quantify major polyphenols in GCE i.e., catechin, epicatechin, E-resveratrol, E-piceatannol, ampelopsin A, E-epsilon-viniferin, Hopeaphenol, isoHopeaphenol, E-miyabenol C and E-vitisin B from selected cultivars. Skin whitening potential through tyrosinase inhibition assay and the activation capacity of cell longevity protein (SIRT1) of GCE were compared to pure E-resveratrol and E-epsilon-viniferin. Drug-likeness of GCE polyphenols were calculated, allowing the prediction of skin permeability and bioavailability. Finally, the present data enabled the consideration of GCE from polyphenol-rich varieties as multifunctional cosmetic ingredients in accordance with green chemistry practices.
A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves.[Pubmed:32397203]
Antioxidants (Basel). 2020 May 8;9(5). pii: antiox9050398.
Due to their biological activities, both in plants and in humans, there is a great interest in finding natural sources of phenolic compounds or ways to artificially manipulate their levels. During the last decade, a significant amount of these compounds has been reported in the vegetative organs of the vine plant. In the roots, woods, canes, stems, and leaves, at least 183 phenolic compounds have been identified, including 78 stilbenes (23 monomers, 30 dimers, 8 trimers, 16 tetramers, and 1 hexamer), 15 hydroxycinnamic acids, 9 hydroxybenzoic acids, 17 flavan-3-ols (of which 9 are proanthocyanidins), 14 anthocyanins, 8 flavanones, 35 flavonols, 2 flavones, and 5 coumarins. There is great variability in the distribution of these chemicals along the vine plant, with leaves and stems/canes having flavonols (83.43% of total phenolic levels) and flavan-3-ols (61.63%) as their main compounds, respectively. In light of the pattern described from the same organs, quercetin-3-O-glucuronide, quercetin-3-O-galactoside, quercetin-3-O-glucoside, and caftaric acid are the main flavonols and hydroxycinnamic acids in the leaves; the most commonly represented flavan-3-ols and flavonols in the stems and canes are catechin, epicatechin, procyanidin B1, and quercetin-3-O-galactoside. The main stilbenes (trans-epsilon-viniferin, trans-resveratrol, isoHopeaphenol/Hopeaphenol, vitisin B, and ampelopsins) accumulate primarily in the woods, followed by the roots, the canes, and the stems, whereas the leaves, which are more exposed to environmental stresses, have a low concentration of these compounds. Data provided in this review could be used as (i) a metabolomic tool for screening in targeted and untargeted analyses and (ii) a reference list in studies aimed at finding ways to induce naturally occurring polyphenols on an industrial scale for pant and human disease control.
Exploring resveratrol dimers as virulence blocking agents - Attenuation of type III secretion in Yersinia pseudotuberculosis and Pseudomonas aeruginosa.[Pubmed:32034212]
Sci Rep. 2020 Feb 7;10(1):2103.
Bacterial infections continue to threaten humankind and the rapid spread of antibiotic resistant bacteria is alarming. Current antibiotics target essential bacterial processes and thereby apply a strong selective pressure on pathogenic and non-pathogenic bacteria alike. One alternative strategy is to block bacterial virulence systems that are essential for the ability to cause disease but not for general bacterial viability. We have previously show that the plant natural product (-)-Hopeaphenol blocks the type III secretion system (T3SS) in the Gram-negative pathogens Yersinia pseudotuberculosis and Pseudomonas aeruginosa. (-)-Hopeaphenol is a resveratrol tetramer and in the present study we explore various resveratrol dimers, including partial structures of (-)-Hopeaphenol, as T3SS inhibitors. To allow rapid and efficient assessment of T3SS inhibition in P. aeruginosa, we developed a new screening method by using a green fluorescent protein reporter under the control of the ExoS promoter. Using a panel of assays we showed that compounds with a benzofuran core structure i.e. viniferifuran, dehydroampelopsin B, anigopreissin A, dehydro-delta-viniferin and resveratrol-piceatannol hybrid displayed significant to moderate activities towards the T3SS in Y. pseudotuberculosis and P. aeruginosa.
Inhibition of the type III secretion system of Pseudomonas syringae pv. tomato DC3000 by resveratrol oligomers identified in Vitis vinifera L.[Pubmed:31994325]
Pest Manag Sci. 2020 Jul;76(7):2294-2303.
BACKGROUND: The bacterial type III secretion system (T3SS) is one of the virulence determinants of Gram-negative bacteria through which various effector and virulence proteins are translocated into host cells. RESULTS: We constructed an assay system to screen inhibitors of hrpA gene expression (a structural gene of Hrp pili) in Pseudomonas syringae pv. tomato DC3000. In a plant extract library screening, the root extract of Vitis vinifera L. displayed the most prominent activity. Three resveratrol oligomers, Hopeaphenol, isoHopeaphenol and ampelopsin A, were identified in grapevine root extract, which significantly reduced the transcription levels of the hrpA, hrpL and hopP1 genes without growth retardation. Additional resveratrol derivatives identified in other plant extracts were also examined for their inhibitory effect on hrpA expression. Another resveratrol oligomer, kobophenol A, also inhibited the transcription of the hrpA gene and other T3SS-related genes, while resveratrol monomers (resveratrol and piceatannol) were not effective. The severity of bacterial specks was reduced by each Hopeaphenol, isoHopeaphenol and ampelopsin A treatment. CONCLUSION: These results show the potential of resveratrol derivatives as anti-virulence agents for the control of plant diseases.
Anti-inflammatory effect and mechanism of action of ellagic acid-3,3',4-trimethoxy-4'-O-alpha-L-rhamnopyranoside isolated from Hopea parviflora in lipopolysaccharide-stimulated RAW 264.7 macrophages.[Pubmed:31711318]
Nat Prod Res. 2019 Nov 12:1-5.
Phytochemical investigation of the stem bark of Hopea parviflora resulted in the isolation of 9 compounds; which includes friedelin (1), friedelin-3beta-ol (2), (-)-ampelopsin A (3), (-)-varepsilon-viniferin (4), (-)-Hopeaphenol (5), vaticaphenol A (6), 2,4,8-trihydroxyphenanthrene-2-O-glucoside (7), ellagic acid-3,3',4-trimethoxy-4'-O-alpha-L-rhamnopyranoside (8) and beta-sitosterol-beta-D-glucoside (9). Among them, compounds 1, 2, 6, 7, 8 and 9 are isolated for the first time from this species. Further, we evaluated the anti-inflammatory activity of compounds 4, 5, 6, 7 and 8. In this study, compound 8 inhibited the activity of proinflammatory mediators like NO, TNF-alpha, IL-6, 5-LOX and COX-2, also promoted the action of anti-inflammatory mediator like IL-10 via inhibition of the NF-kappaB pathway in LPS-stimulated RAW 264.7 macrophages.
Isolation and characterization of resveratrol oligomers from the stem bark of Hopea ponga (Dennst.) Mabb. And their antidiabetic effect by modulation of digestive enzymes, protein glycation and glucose uptake in L6 myocytes.[Pubmed:30844488]
J Ethnopharmacol. 2019 May 23;236:196-204.
ETHNOPHARMACOLOGICAL RELEVANCE: Hopea ponga (Dennst.) Mabb. Is used in traditional herbal formulations for diabetes complications. The aim of this study is to evaluate the antidiabetic effect of extracts and compounds from H. ponga. MATERIALS AND METHODS: Silica gel column chromatography was performed to identify various chemical components of the plant extract. Different extracts of H. ponga and isolated compounds were screened for their antidiabetic effect by modulation of digestive enzymes and protein glycation. The effect of glucose uptake by the compounds and the pathways through which the compounds mediate the glucose uptake potential were confirmed by fluorescent microscopy, flow cytometry and western blot analysis. RESULTS: Acetone and ethanol extracts of the stem bark of Hopea ponga (Dennst.) Mabb. Afforded six resveratrol oligomers namely, E-resveratrol (1), (-)-epsilon-viniferin (2), (-)-alpha-viniferin (3), trihydroxyphenanthrene glucoside (THPG) (4), vaticaphenol A (5), (-)-Hopeaphenol (6), along with four phytosterols. The structures were determined on the basis of spectroscopic analyses including nuclear magnetic resonance (NMR) spectroscopy and high resolution mass spectrometry (HRMS) data. Compounds 1-5 and 7-10 were tested for their alpha-glucosidase, alpha-amylase and glycation inhibitiory activities. All the resveratrol oligomers (1-5) showed prominent alpha-glucosidase inhibition with IC50 values, 12.56+/-1.00, 23.98+/-1.11, 7.17+/-1.10, 31.74+/-0.42 and 16.95+/-0.39muM, respectively. Molecular docking studies also supported the observed alpha-glucosidase inhibition. Compound 3 displayed IC50 values of 4.85+/-0.06 and 27.10+/-0.04muM in alpha-amylase and glycation inhibitory assays activity. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay revealed that the compounds 3 and 4 were found to be less toxic at a concentration of 100muM (<10%) and 25muM (<20%), respectively. The effect of glucose uptake performed by 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) in L6 myoblast were measured by fluorescent microscopy and flow cytometry. The compounds 3 and 4 showed 2-NBDG uptake of 49.6% and 38.8% respectively. By examining the molecular pathway through which the compounds elicit their glucose uptake potential, it was observed that both the compounds mainly act via AMPK pathway. CONCLUSION: This is the first report on the isolation of compounds from H. ponga. Altogether, the results of this study reveal the antidiabetic effects of H. ponga extracts and isolated compounds promoting traditional use of this plant in the treatment of diabetes.
Structural analysis of the inhibitory effects of polyphenols, (+)-hopeaphenol and (-)-isohopeaphenol, on human SIRT1.[Pubmed:30537158]
Biofactors. 2019 Mar;45(2):253-258.
Human sirtuin 1 (hSIRT1) is a NAD(+) -dependent deacetylase that regulates several cellular processes. Unlike resveratrol, natural polymeric phenolic compounds isolated from Vitaceae are mostly hSIRT1 inhibitors. The resveratrol tetramer, (+)-Hopeaphenol ((+)-HP), and its geometric isomer, (-)-isoHopeaphenol ((-)-iHP), were tested for inhibitory effects on purified hSIRT1 using a fluorescent derivative of peptide substrate p53-AMC (Fluor de Lys) and a cofactor NAD(+) . The Lineweaver-Burk plots indicated that both (+)-HP and (-)-iHP were competitive inhibitors against NAD(+) . Computer-assisted modeling of the binding of these molecules with hSIRT1 protein provided the most feasible conformation of the enzyme-inhibitor complex. (c) 2018 BioFactors, 45(2):253-258, 2019.
Antibacterial Activities of Metabolites from Vitis rotundifolia (Muscadine) Roots against Fish Pathogenic Bacteria.[Pubmed:30366372]
Molecules. 2018 Oct 25;23(11). pii: molecules23112761.
Enteric septicemia of catfish, columnaris disease and streptococcosis, caused by Edwardsiella ictaluri, Flavobacterium columnare and Streptococcus iniae, respectively, are the most common bacterial diseases of economic significance to the pond-raised channel catfish Ictalurus punctatus industry. Certain management practices are used by catfish farmers to prevent large financial losses from these diseases such as the use of commercial antibiotics. In order to discover environmentally benign alternatives, using a rapid bioassay, we evaluated a crude extract from the roots of muscadine Vitis rotundifolia against these fish pathogenic bacteria and determined that the extract was most active against F. columnare. Subsequently, several isolated compounds from the root extract were isolated. Among these isolated compounds, (+)-Hopeaphenol (2) and (+)-vitisin A (3) were found to be the most active (bacteriostatic activity only) against F. columnare, with 24-h 50% inhibition concentrations of 4.0 +/- 0.7 and 7.7 +/- 0.6 mg/L, respectively, and minimum inhibitory concentrations of 9.1 +/- 0 mg/L for each compound which were approximately 25X less active than the drug control florfenicol. Efficacy testing of 2 and 3 is necessary to further evaluate the potential for these compounds to be used as antibacterial agents for managing columnaris disease.
Using the relative abundance of characteristic product ions in UHPLC-ESI-QTOF-MS(2) methods to identify isomers of resveratrol oligomers in extracts of Xinjiang winegrape stems.[Pubmed:30149299]
J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Oct 1;1096:88-94.
Stilbenoids, particularly resveratrol and its oligomer, are abundantly present in grapes, and their antioxidant activities have been widely reported. A quick and simple method based on UHPLC-ESI-QTOF-MS(2) was established for the fragmentation pathways analysis of trans-epsilon-Viniferin, cis-epsilon-Viniferin, trans-delta-Viniferin and (-)-Hopeaphenol. MS/MS experiments on the [M-H](-) ions provided abundant structural information, especially regarding the relative abundance of the key product ion at m/z 347. The product ion was used to further identify structures in isomers of resveratrol dimers and its analogues. Based on the fragmentation pathways, we tentatively determined two compounds from the crude extracts of Xinjiang winegrape stems as Gnetin C and cis-Scirpusin A. Results from these experiments contribute to a more complete understanding of the stilbene compounds found in grape stems. The UHPLC-QTOF-MS(2) method can be used for the rapid analysis of stilbenes compounds in plant materials, foods and wine.
Phenolic Compounds from Belamcanda chinensis Seeds.[Pubmed:29510567]
Molecules. 2018 Mar 5;23(3). pii: molecules23030580.
Two new sucrose derivatives, namely, belamcanosides A (1) and B (2), together with five other known compounds (3-7), were isolated from the seeds of Belamcanda chinensis (L.) DC. Their structures were identified based on spectroscopic data. Especially, the absolute configurations of fructose and glucose residues in 1 and 2 were assigned by acid hydrolysis, followed by derivatization and gas chromatography (GC) analysis. Among the known compounds, (-)-Hopeaphenol (3), (+)-syringaresinol (4), and quercetin (5), were isolated from B. chinensis for the first time. In addition, biological evaluation of 1 and 2 against cholesterol synthesis and metabolism at the gene level was carried out. The results showed that compounds 1 and 2 could regulate the expression of cholesterol synthesis and metabolism-associated genes, including 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), squalene epoxidase (SQLE), low density lipoprotein receptor (LDLR), and sortilin (SORT1) genes in HepG2 cells.
C18 core-shell column with in-series absorbance and fluorescence detection for simultaneous monitoring of changes in stilbenoid and proanthocyanidin concentrations during grape cane storage.[Pubmed:29331860]
J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Feb 1;1074-1075:70-78.
Grape canes, the residues from the annual pruning of vines, contain high levels of inducible (E)-resveratrol and also oligomeric stilbenoids and proanthocyanidins. These two families of phenolic compounds are bioactive, but to quantify them in a single chromatographic run using only ultraviolet detection is a difficult task. To overcome this limitation, a chromatographic method was developed using a core shell column for separation, an ultraviolet-visible diode array detector (DAD) and a fluorescence (FL) detector connected in series for quantification, with an electrospray ionization interface (ESI) and a triple quadrupole mass spectrometric detector (MS/MS) added for identification of the analytes. The proanthocyanidins (+)-catechin, (-)-epicatechin, procyanidins B1, B2, and C1, an unknown dimer and trimer, two prodelphinidin dimers, and monogallate procyanidin dimers were detected in the tested grape cane samples. The stilbenoids detected were (E)-resveratrol, (E)-piceatannol, (E)-piceid, (E)-epsilon-viniferin, vitisin B, a glycosylated monomer, three oxidized dimers, an unknown dimer and a tetramer, pallidol, Hopeaphenol, (E)-delta-viniferin, and (E)-omega-viniferin. However, this method required 60min for each analysis. A faster and more efficient method for quantitative analysis was developed based on HPLC-DAD-FL, reducing the time required to 24min for the simultaneous quantification of proanthocyanidins and stilbenoids in Cabernet Sauvignon, Pinot Noir, and Tintorera grape canes stored at controlled temperatures and relativity humidities for 134days after pruning. To the best of our knowledge, this is the first time a prodelphinidin dimer has been quantified in grape canes. The incorporation of fluorescence detection in series with DAD not only allowed the quantification of proanthocyanidins, it also improved the detectability of some minor stilbenoids present in the canes, such as (E)-piceid. The (E)-resveratrol and (E)-piceatannol levels increased significantly during cane storage, while those of (E)-epsilon-viniferin and ampelopsin A did not show significant increases. The relative humidity had a determining effect on the levels of (E)-resveratrol and (E)-piceatannol in the canes of all varieties studied; their concentrations were higher at a relative humidity of 60% than at 70%. This is the first time that the proanthocyanidin profiles of canes stored after pruning were monitored under controlled conditions of temperature, time and relative humidity. The concentration of (-)-epicatechin decreased during storage under both relative humidities. Furthermore, the levels of proanthocyanidin B1 and the prodelphinidin dimer also decreased to a certain extent.


