IsohopeaphenolCAS# 197446-77-8 |
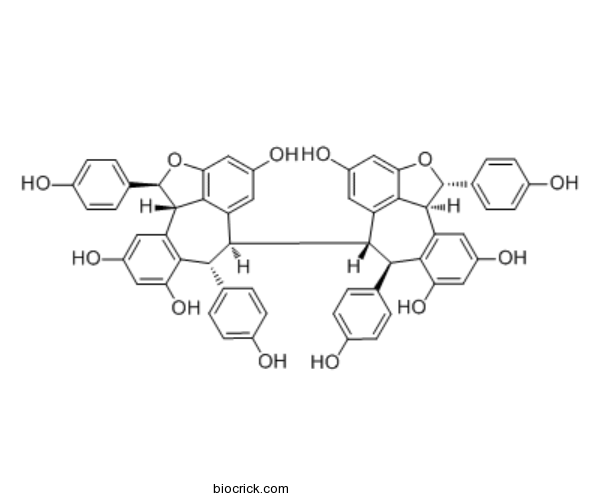
- Hopeaphenol
Catalog No.:BCN9570
CAS No.:388582-37-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 197446-77-8 | SDF | Download SDF |
| PubChem ID | 13844644 | Appearance | Brown powder |
| Formula | C56H42O12 | M.Wt | 906.9 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | (+)-Isohopeaphenol;17912-85-5 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,8S,9R,16R)-8,16-bis(4-hydroxyphenyl)-9-[(1R,8S,9R,16R)-4,6,12-trihydroxy-8,16-bis(4-hydroxyphenyl)-15-oxatetracyclo[8.6.1.02,7.014,17]heptadeca-2(7),3,5,10(17),11,13-hexaen-9-yl]-15-oxatetracyclo[8.6.1.02,7.014,17]heptadeca-2(7),3,5,10(17),11,13-hexaene-4,6,12-triol | ||
| SMILES | C1=CC(=CC=C1C2C(C3=C4C(C(OC4=CC(=C3)O)C5=CC=C(C=C5)O)C6=C2C(=CC(=C6)O)O)C7C(C8=C(C=C(C=C8O)O)C9C(OC1=CC(=CC7=C91)O)C1=CC=C(C=C1)O)C1=CC=C(C=C1)O)O | ||
| Standard InChIKey | YQQUILZPDYJDQJ-VHQOTMCHSA-N | ||
| Standard InChI | InChI=1S/C56H42O12/c57-29-9-1-25(2-10-29)45-47-37(17-33(61)21-41(47)65)53-49-39(19-35(63)23-43(49)67-55(53)27-5-13-31(59)14-6-27)51(45)52-40-20-36(64)24-44-50(40)54(56(68-44)28-7-15-32(60)16-8-28)38-18-34(62)22-42(66)48(38)46(52)26-3-11-30(58)12-4-26/h1-24,45-46,51-66H/t45-,46-,51-,52-,53+,54+,55-,56-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isohopeaphenol Dilution Calculator

Isohopeaphenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1027 mL | 5.5133 mL | 11.0266 mL | 22.0531 mL | 27.5664 mL |
| 5 mM | 0.2205 mL | 1.1027 mL | 2.2053 mL | 4.4106 mL | 5.5133 mL |
| 10 mM | 0.1103 mL | 0.5513 mL | 1.1027 mL | 2.2053 mL | 2.7566 mL |
| 50 mM | 0.0221 mL | 0.1103 mL | 0.2205 mL | 0.4411 mL | 0.5513 mL |
| 100 mM | 0.011 mL | 0.0551 mL | 0.1103 mL | 0.2205 mL | 0.2757 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hopeaphenol
Catalog No.:BCN9570
CAS No.:388582-37-4
- Myricetin 3,7,3'-trimethyl ether 5'-O-glucoside
Catalog No.:BCN9569
CAS No.:2170444-56-9
- 3,4-Dihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9568
CAS No.:454473-97-3
- 3,4,5-Trihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9567
CAS No.:2172431-63-7
- Pukateine
Catalog No.:BCN9566
CAS No.:81-67-4
- 11-Methylforsythide
Catalog No.:BCN9565
CAS No.:159598-00-2
- Adoxoside
Catalog No.:BCN9564
CAS No.:42830-26-2
- 3-O-Methylellagic acid 4-O-rhamnoside
Catalog No.:BCN9563
CAS No.:639089-97-7
- Quercetin 3,5,3'-trimethyl ether
Catalog No.:BCN9562
CAS No.:13459-09-1
- Aesculioside C
Catalog No.:BCN9561
CAS No.:254896-65-6
- Isoaesculioside D
Catalog No.:BCN9560
CAS No.:1184581-59-6
- Sonderianol
Catalog No.:BCN9559
CAS No.:85563-65-1
- 1,7-Bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one
Catalog No.:BCN9572
CAS No.:149732-52-5
- Taraxinic acid
Catalog No.:BCN9573
CAS No.:75911-33-0
- Harringtonolide
Catalog No.:BCN9574
CAS No.:64761-48-4
- Caryatin
Catalog No.:BCN9575
CAS No.:1486-66-4
- Demethylagrimonolide 6-O-glucoside
Catalog No.:BCN9576
CAS No.:1257408-55-1
- Momordicin IV
Catalog No.:BCN9577
CAS No.:894412-35-2
- Taikuguasin D
Catalog No.:BCN9578
CAS No.:1627163-80-7
- Karaviloside XI
Catalog No.:BCN9579
CAS No.:934739-35-2
- Hainanolidol
Catalog No.:BCN9580
CAS No.:73213-63-5
- Fortunolide A
Catalog No.:BCN9581
CAS No.:252574-51-9
- Actinodaphnine
Catalog No.:BCN9582
CAS No.:517-69-1
- Goyaglycoside d
Catalog No.:BCN9583
CAS No.:333332-50-6
Grape Cane Extracts as Multifunctional Rejuvenating Cosmetic Ingredient: Evaluation of Sirtuin Activity, Tyrosinase Inhibition and Bioavailability Potential.[Pubmed:32397228]
Molecules. 2020 May 8;25(9). pii: molecules25092203.
Grape canes are waste biomass of viticulture containing bioactive polyphenols valuable in cosmetics. Whereas several studies reported the cosmetic activities of E-resveratrol, only few described the potential of E-epsilon-viniferin, the second major constituent of grape cane extracts (GCE), and none of them investigated GCE as a natural blend of polyphenols for cosmetic applications. In this study, we considered the potential of GCE from polyphenol-rich grape varieties as multifunctional cosmetic ingredients. HPLC analysis was performed to quantify major polyphenols in GCE i.e., catechin, epicatechin, E-resveratrol, E-piceatannol, ampelopsin A, E-epsilon-viniferin, hopeaphenol, Isohopeaphenol, E-miyabenol C and E-vitisin B from selected cultivars. Skin whitening potential through tyrosinase inhibition assay and the activation capacity of cell longevity protein (SIRT1) of GCE were compared to pure E-resveratrol and E-epsilon-viniferin. Drug-likeness of GCE polyphenols were calculated, allowing the prediction of skin permeability and bioavailability. Finally, the present data enabled the consideration of GCE from polyphenol-rich varieties as multifunctional cosmetic ingredients in accordance with green chemistry practices.
A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves.[Pubmed:32397203]
Antioxidants (Basel). 2020 May 8;9(5). pii: antiox9050398.
Due to their biological activities, both in plants and in humans, there is a great interest in finding natural sources of phenolic compounds or ways to artificially manipulate their levels. During the last decade, a significant amount of these compounds has been reported in the vegetative organs of the vine plant. In the roots, woods, canes, stems, and leaves, at least 183 phenolic compounds have been identified, including 78 stilbenes (23 monomers, 30 dimers, 8 trimers, 16 tetramers, and 1 hexamer), 15 hydroxycinnamic acids, 9 hydroxybenzoic acids, 17 flavan-3-ols (of which 9 are proanthocyanidins), 14 anthocyanins, 8 flavanones, 35 flavonols, 2 flavones, and 5 coumarins. There is great variability in the distribution of these chemicals along the vine plant, with leaves and stems/canes having flavonols (83.43% of total phenolic levels) and flavan-3-ols (61.63%) as their main compounds, respectively. In light of the pattern described from the same organs, quercetin-3-O-glucuronide, quercetin-3-O-galactoside, quercetin-3-O-glucoside, and caftaric acid are the main flavonols and hydroxycinnamic acids in the leaves; the most commonly represented flavan-3-ols and flavonols in the stems and canes are catechin, epicatechin, procyanidin B1, and quercetin-3-O-galactoside. The main stilbenes (trans-epsilon-viniferin, trans-resveratrol, Isohopeaphenol/hopeaphenol, vitisin B, and ampelopsins) accumulate primarily in the woods, followed by the roots, the canes, and the stems, whereas the leaves, which are more exposed to environmental stresses, have a low concentration of these compounds. Data provided in this review could be used as (i) a metabolomic tool for screening in targeted and untargeted analyses and (ii) a reference list in studies aimed at finding ways to induce naturally occurring polyphenols on an industrial scale for pant and human disease control.
Inhibition of the type III secretion system of Pseudomonas syringae pv. tomato DC3000 by resveratrol oligomers identified in Vitis vinifera L.[Pubmed:31994325]
Pest Manag Sci. 2020 Jul;76(7):2294-2303.
BACKGROUND: The bacterial type III secretion system (T3SS) is one of the virulence determinants of Gram-negative bacteria through which various effector and virulence proteins are translocated into host cells. RESULTS: We constructed an assay system to screen inhibitors of hrpA gene expression (a structural gene of Hrp pili) in Pseudomonas syringae pv. tomato DC3000. In a plant extract library screening, the root extract of Vitis vinifera L. displayed the most prominent activity. Three resveratrol oligomers, hopeaphenol, Isohopeaphenol and ampelopsin A, were identified in grapevine root extract, which significantly reduced the transcription levels of the hrpA, hrpL and hopP1 genes without growth retardation. Additional resveratrol derivatives identified in other plant extracts were also examined for their inhibitory effect on hrpA expression. Another resveratrol oligomer, kobophenol A, also inhibited the transcription of the hrpA gene and other T3SS-related genes, while resveratrol monomers (resveratrol and piceatannol) were not effective. The severity of bacterial specks was reduced by each hopeaphenol, Isohopeaphenol and ampelopsin A treatment. CONCLUSION: These results show the potential of resveratrol derivatives as anti-virulence agents for the control of plant diseases.
Structural analysis of the inhibitory effects of polyphenols, (+)-hopeaphenol and (-)-isohopeaphenol, on human SIRT1.[Pubmed:30537158]
Biofactors. 2019 Mar;45(2):253-258.
Human sirtuin 1 (hSIRT1) is a NAD(+) -dependent deacetylase that regulates several cellular processes. Unlike resveratrol, natural polymeric phenolic compounds isolated from Vitaceae are mostly hSIRT1 inhibitors. The resveratrol tetramer, (+)-hopeaphenol ((+)-HP), and its geometric isomer, (-)-Isohopeaphenol ((-)-iHP), were tested for inhibitory effects on purified hSIRT1 using a fluorescent derivative of peptide substrate p53-AMC (Fluor de Lys) and a cofactor NAD(+) . The Lineweaver-Burk plots indicated that both (+)-HP and (-)-iHP were competitive inhibitors against NAD(+) . Computer-assisted modeling of the binding of these molecules with hSIRT1 protein provided the most feasible conformation of the enzyme-inhibitor complex. (c) 2018 BioFactors, 45(2):253-258, 2019.
Antioxidant and Cytoprotective Activities of Grapevine Stilbenes.[Pubmed:28551990]
J Agric Food Chem. 2017 Jun 21;65(24):4952-4960.
Grapevine stem extracts are viticulture byproducts rich in stilbenes that are increasingly studied for their potential biological activities. This study aimed to investigate some biological activities of a grape byproduct with high stilbenoid content and to point out the molecules responsible of these beneficial activities. As a consequence, the extract was subjected to a bioguided fractionation and separation by centrifugal partition chromatography. The obtained fractions were characterized by liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance. Fractions were purified further by column chromatography and resulted in the purification of the main constituents. Thirteen stilbenes have been quantified. The most abundant compounds were epsilon-viniferin, resveratrol, and, in lesser amounts, Isohopeaphenol and ampelopsin A. The extract, fractions, and major stilbenes were tested for their antioxidant activity by oxygen radical absorbance capacity and their cyprotective effects against beta-amyloid on rat pheochromocytoma cells. Among them, fraction 5 showed significant antioxidant activity and fraction 2 had a significant cytoprotective effect against beta-amyloid-induced toxicity. Two putative inhibitors of beta-amyloid toxicity have been identified: ampelopsin A and piceatannol.
Stilbenes from Vitis vinifera L. Waste: A Sustainable Tool for Controlling Plasmopara Viticola.[Pubmed:28288509]
J Agric Food Chem. 2017 Apr 5;65(13):2711-2718.
Stilbene-enriched extracts from Vitis vinifera waste (cane, wood, and root) were characterized by UHPLC-MS. Eleven stilbenes were identified and quantified as follows: ampelopsin A, (E)-piceatannol, pallidol, (E)-resveratrol, hopeaphenol, Isohopeaphenol, (E)-epsilon-viniferin, (E)-miyabenol C, (E)-omega-viniferin, r2-viniferin, and r-viniferin. The fungicide concentration inhibiting 50% of growth of Plasmopara viticola sporulation (IC50) was determined for the extracts and also for the main compounds isolated. r-Viniferin followed by hopeaphenol and r2-viniferin showed low IC50 and thus high efficacy against Plasmopara viticola. Regarding stilbene extracts, wood extract followed by root extract showed the highest antifungal activities. These data suggest that stilbene complex mixtures from Vitis vinifera waste could be used as a cheap source of bioactive stilbenes for the development of natural fungicides.
Quantitative Determination of Stilbenoids and Dihydroisocoumarins in Shorea roxburghii and Evaluation of Their Hepatoprotective Activity.[Pubmed:28230758]
Int J Mol Sci. 2017 Feb 20;18(2). pii: ijms18020451.
A simultaneous quantitative analytical method for 13 stilbenoids including (-)-hopeaphenol (1), (+)-Isohopeaphenol (2), hemsleyanol D (3), (-)-ampelopsin H (4), vaticanols A (5), E (6), and G (7), (+)-alpha-viniferin (8), pauciflorol A (9), hopeafuran (10), (-)-balanocarpol (11), (-)-ampelopsin A (12), and trans-resveratrol 10-C-beta-d-glucopyranoside (13), and two dihydroisocoumarins, phayomphenols A(1) (14) and A(2) (15) in the extract of Shorea roxburghii (dipterocarpaceae) was developed. According to the established protocol, distributions of these 15 polyphenols (1-15) in the bark and wood parts of S. roxburghii and a related plant Cotylelobium melanoxylon were evaluated. In addition, the principal polyphenols (1, 2, 8, 13-15) exhibited hepatoprotective effects against d-galactosamine (d-galN)/lipopolysaccharide (LPS)-induced liver injury in mice at a dose of 100 or 200 mg/kg, p.o. To characterize the mechanisms of action, the isolates were examined in in vitro studies assessing their effects on (i) d-GalN-induced cytotoxicity in primary cultured mouse hepatocytes; (ii) LPS-induced nitric oxide (NO) production in mouse peritoneal macrophages; and (iii) tumor necrosis factor-alpha (TNF-alpha)-induced cytotoxicity in L929 cells. The mechanisms of action of these polyphenols (1, 2, and 8) were suggested to be dependent on the inhibition of LPS-induced macrophage activation and reduction of sensitivity of hepatocytes to TNF-alpha. However, none of the isolates reduced the cytotoxicity caused by d-GalN.
Piceatannol and Other Wine Stilbenes: A Pool of Inhibitors against alpha-Synuclein Aggregation and Cytotoxicity.[Pubmed:27314384]
Nutrients. 2016 Jun 15;8(6). pii: nu8060367.
The aggregation of alpha-synuclein is one on the key pathogenic events in Parkinson's disease. In the present study, we investigated the inhibitory capacities of stilbenes against alpha-synuclein aggregation and toxicity. Thioflavin T fluorescence, transmission electronic microscopy, and SDS-PAGE analysis were performed to investigate the inhibitory effects of three stilbenes against alpha-synuclein aggregation: piceatannol, ampelopsin A, and Isohopeaphenol. Lipid vesicle permeabilization assays were performed to screen stilbenes for protection against membrane damage induced by aggregated alpha-synuclein. The viability of PC12 cells was examined using an MTT assay to assess the preventive effects of stilbenes against alpha-synuclein-induced toxicity. Piceatannol inhibited the formation of alpha synuclein fibrils and was able to destabilize preformed filaments. It seems to induce the formation of small soluble complexes protecting membranes against alpha-synuclein-induced damage. Finally, piceatannol protected cells against alpha-synuclein-induced toxicity. The oligomers tested (ampelopsin A and hopeaphenol) were less active.
Composition and Tissue-Specific Distribution of Stilbenoids in Grape Canes Are Affected by Downy Mildew Pressure in the Vineyard.[Pubmed:26373576]
J Agric Food Chem. 2015 Sep 30;63(38):8472-7.
Grape canes are byproducts of viticulture containing valuable bioactive stilbenoids including monomers and oligomers of E-resveratrol. Although effective contents in stilbenoids are known to be highly variable, the determining factors influencing this composition remain poorly understood. As stilbenoids are locally induced defense compounds in response to phytopathogens, this study assessed the impact of downy mildew infection during the growing season on the stilbenoid composition of winter-harvested grape canes. The spatial distribution between pith, conducting tissues, and cortex of E-piceatannol, E-resveratrol, E-epsilon-viniferin, ampelopsin A, E-miyabenol C, Z/E-vitisin B, hopeaphenol, and Isohopeaphenol in grape canes from infected vineyards was strongly altered. In conducting tissues, representing the main site of stilbenoid accumulation, E-epsilon-viniferin content was higher and E-resveratrol content was lower. These findings suppose that the health status in vineyards could modify the composition of stilbenoids in winter-harvested grape canes and subsequently the potential biological properties of the valuable extracts.
Natural stilbenoids isolated from grapevine exhibiting inhibitory effects against HIV-1 integrase and eukaryote MOS1 transposase in vitro activities.[Pubmed:24312275]
PLoS One. 2013 Nov 28;8(11):e81184.
Polynucleotidyl transferases are enzymes involved in several DNA mobility mechanisms in prokaryotes and eukaryotes. Some of them such as retroviral integrases are crucial for pathogenous processes and are therefore good candidates for therapeutic approaches. To identify new therapeutic compounds and new tools for investigating the common functional features of these proteins, we addressed the inhibition properties of natural stilbenoids deriving from resveratrol on two models: the HIV-1 integrase and the eukaryote MOS-1 transposase. Two resveratrol dimers, leachianol F and G, were isolated for the first time in Vitis along with fourteen known stilbenoids: E-resveratrol, E-piceid, E-pterostilbene, E-piceatannol, (+)-E-epsilon-viniferin, E-epsilon-viniferinglucoside, E-scirpusin A, quadragularin A, ampelopsin A, pallidol, E-miyabenol C, E-vitisin B, hopeaphenol, and Isohopeaphenol and were purified from stalks of Vitis vinifera (Vitaceae), and moracin M from stem bark of Milliciaexelsa (Moraceae). These compounds were tested in in vitro and in vivo assays reproducing the activity of both enzymes. Several molecules presented significant inhibition on both systems. Some of the molecules were found to be active against both proteins while others were specific for one of the two models. Comparison of the differential effects of the molecules suggested that the compounds could target specific intermediate nucleocomplexes of the reactions. Additionally E-pterostilbene was found active on the early lentiviral replication steps in lentiviruses transduced cells. Consequently, in addition to representing new original lead compounds for further modelling of new active agents against HIV-1 integrase, these molecules could be good tools for identifying such reaction intermediates in DNA mobility processes.
Chemical dereplication of wine stilbenoids using high performance liquid chromatography-nuclear magnetic resonance spectroscopy.[Pubmed:23566915]
J Chromatogr A. 2013 May 10;1289:19-26.
Wine is a major dietary source of numerous potentially health promoting stilbenoids that have been the subject of many qualitative and quantitative studies. However, our initial HPLC-MS analyses of crude wine samples demonstrated the presence of compounds with molecular weights matching characteristic stilbenoid dimers, trimers, and tetramers that were unaccounted for in the literature. Due to the likelihood that these are known compounds, a chemical dereplication method is highly desirable. We developed such a method using LC-DAD-MS monitored fractionation steps, using adsorption and centrifugal partition chromatography (CPC), to obtain fractions rich in stilbenoids for analysis in stopped-flow LC-NMR. (1)H NMR spectra and MS data were cross-referenced with our laboratory database and the literature for identification. This method yielded highly useful structural information, allowing the characterization of previously unidentified stilbenoids in wine, ampelopsin C, Isohopeaphenol, quadrangularin A, and E-omega-viniferin. These results demonstrate the usefulness of stop-flow LC-NMR in conjunction with LC-MS guided fractionation for the dereplication of compounds of interest in general, and specifically for expanding the current knowledge of wine chemistry.
Phenolics and their antifungal role in grapevine wood decay: focus on the Botryosphaeriaceae family.[Pubmed:23145924]
J Agric Food Chem. 2012 Dec 5;60(48):11859-68.
The interaction between Vitis vinifera and trunk disease fungi requires better understanding. We studied the role of phenolics as possible plant defense compounds in this context. The impact of 24 grapevine phenolic compounds was determined on 6 major wood decay fungi by an in vitro agar plate assay. Hydroxystilbenoids, especially oligomers such as miyabenol C, Isohopeaphenol, and vitisin A and B, greatly reduced the growth of the fungi, except that of Phaeoacremonium aleophilum . A detailed investigation in 10 Botryosphaeriaceae strains revealed that all of the studied members of this family display a common susceptibility to phenolics that is more or less significant. Then we undertook a quantitative analysis of stilbenoid content in grapevine plantlets inoculated with Botryosphaeriaceae to investigate whether in planta these fungi have to counteract the most active phenolics. On the basis of our results, the possible role of phenolics in grapevine defense against trunk disease agents is discussed.
Anti-hyperlipidemic constituents from the bark of Shorea roxburghii.[Pubmed:22261856]
J Nat Med. 2012 Jul;66(3):516-24.
The methanol extract from the bark of Shorea roxburghii (Dipterocarpaceae, "Phayom" in Thai) was found to suppress plasma triglyceride elevation in olive oil-treated mice, and also to inhibit pancreatic lipase activity (IC(50) = 31.6 mug/ml). From the extract, two new 3-acetyl-4-phenyl-3,4-dihydroisocoumarins, phayomphenols A(1) (1) and A(2) (2) were isolated, together with 22 known compounds. The structures of 1 and 2 were elucidated on the basis of chemical and spectroscopic evidence, including X-ray crystallographic analysis. Among the isolates, several oligostilbenoids, including (-)-hopeaphenol (3) and (+)-Isohopeaphenol (4), showed inhibitory effects on plasma triglyceride elevation at a dose of 200 mg/kg p.o. and pancreatic lipase inhibitory activity (IC(50) = 32.9 and 26.5 muM, respectively).


