SinococulineCAS# 109351-36-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 109351-36-2 | SDF | Download SDF |
| PubChem ID | 5489400 | Appearance | Powder |
| Formula | C18H23NO5 | M.Wt | 333.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
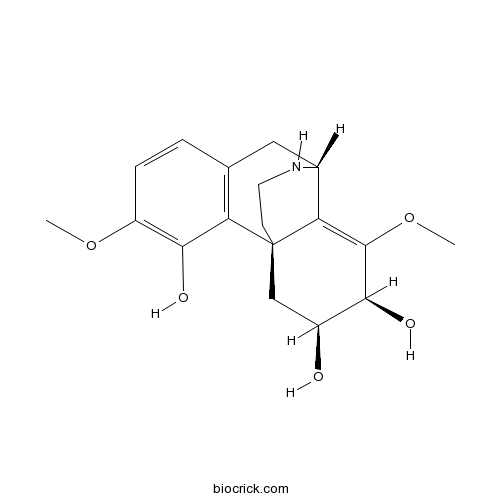
| Chemical Name | (1S,9S,12S,13S)-4,11-dimethoxy-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5,10-tetraene-3,12,13-triol | ||
| SMILES | COC1=C(C2=C(CC3C4=C(C(C(CC42CCN3)O)O)OC)C=C1)O | ||
| Standard InChIKey | MFKPWBJXKCSPGK-KNORBDTNSA-N | ||
| Standard InChI | InChI=1S/C18H23NO5/c1-23-12-4-3-9-7-10-14-17(24-2)15(21)11(20)8-18(14,5-6-19-10)13(9)16(12)22/h3-4,10-11,15,19-22H,5-8H2,1-2H3/t10-,11-,15-,18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sinococuline Dilution Calculator

Sinococuline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9994 mL | 14.997 mL | 29.994 mL | 59.988 mL | 74.985 mL |
| 5 mM | 0.5999 mL | 2.9994 mL | 5.9988 mL | 11.9976 mL | 14.997 mL |
| 10 mM | 0.2999 mL | 1.4997 mL | 2.9994 mL | 5.9988 mL | 7.4985 mL |
| 50 mM | 0.06 mL | 0.2999 mL | 0.5999 mL | 1.1998 mL | 1.4997 mL |
| 100 mM | 0.03 mL | 0.15 mL | 0.2999 mL | 0.5999 mL | 0.7499 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tannagine
Catalog No.:BCN9596
CAS No.:123750-34-5
- Junosine
Catalog No.:BCN9595
CAS No.:103956-34-9
- Citrusinine I
Catalog No.:BCN9594
CAS No.:86680-32-2
- 6a,7-Dehydroboldine
Catalog No.:BCN9593
CAS No.:91599-23-4
- Boschnaloside
Catalog No.:BCN9592
CAS No.:72963-55-4
- Taccaoside E
Catalog No.:BCN9591
CAS No.:1858199-00-4
- 3'-Hydroxy-3,5,8,4',5'-pentamethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN9590
CAS No.:82668-94-8
- 11β,13-Dihydrotaraxinic acid
Catalog No.:BCN9589
CAS No.:1274668-83-5
- Isostephodeline
Catalog No.:BCN9588
CAS No.:56648-85-2
- Blumenol B 9-O-glucoside
Catalog No.:BCN9587
CAS No.:114226-08-3
- Runanine
Catalog No.:BCN9586
CAS No.:100485-12-9
- (+)-Isoampelopsin F
Catalog No.:BCN9585
CAS No.:354553-38-1
- (2R,3R)-Glucodistylin
Catalog No.:BCN9598
CAS No.:27297-45-6
- 3β-Hydroxy-7β,25-dimethoxycucurbita-5,23-dien-19-al
Catalog No.:BCN9599
CAS No.:85372-69-6
- Norushinsunine
Catalog No.:BCN9600
CAS No.:3175-84-6
- 16-Oxoserratenediol
Catalog No.:BCN9601
CAS No.:24513-52-8
- Aquilegiolide
Catalog No.:BCN9602
CAS No.:94481-79-5
- Menisdaurilide
Catalog No.:BCN9603
CAS No.:67765-59-7
- 7,7'-Dihydrotaiwanin C
Catalog No.:BCN9604
CAS No.:216955-79-2
- Stepharine
Catalog No.:BCN9605
CAS No.:2810-21-1
- Taiwanin C
Catalog No.:BCN9606
CAS No.:14944-34-4
- Beiwutine
Catalog No.:BCN9607
CAS No.:76918-93-9
- Apigenin 7-O-(2'',6''-di-O-E-p-coumaroyl)glucoside
Catalog No.:BCN9608
CAS No.:1448779-19-8
- Karavilagenin F
Catalog No.:BCN9609
CAS No.:1639024-15-9
6,7-di-O-acetylsinococuline (FK-3000) induces G2/M phase arrest in breast carcinomas through p38 MAPK phosphorylation and CDC25B dephosphorylation.[Pubmed:25384584]
Int J Oncol. 2015 Feb;46(2):578-86.
We evaluated the cytostatic effect of 6,7-di-O-acetyl-Sinococuline (FK-3000) isolated from Stephania delavayi Diels. against breast carcinoma cell lines MDA-MB231 and MCF-7. FK-3000 suppressed CDC25B phosphorylation directly and indirectly via p38 MAPK phosphorylation. CDC25B dephosphorylation decreased levels of cyclin B and phospho-CDC-2, and ultimately induced cell cycle arrest at the G2/M phase. The p38 MAPK inhibitor, SB 239063 blocked FK-3000-induced p38 MAPK phosphorylation and nuclear accumulation, but did not completely rescue cell death. Conclusively FK-3000 exerts its antiproliferative effect through two pathways: i) G2/M cell cycle arrest via downregulation of cyclin B and phospho-CDC2 by p38 MAPK phosphorylation and CDC25B dephosphorylation, and ii) p38 MAPK-independent induction of apoptosis.
Isolation and antimalarial activity of new morphinan alkaloids on Plasmodium yoelii liver stage.[Pubmed:18456502]
Bioorg Med Chem. 2008 Jun 1;16(11):6186-92.
Decoction of Strychnopsis thouarsii is used in the Malagasy traditional medicine to combat malaria. We have shown that this traditional remedy prevents malaria infection by targeting Plasmodium at its early liver stage. Bioassay-guided fractionation of S. thouarsii stem barks extracts, using a rodent Plasmodium yoelii liver stage parasites inhibition assay, led to isolate the new morphinan alkaloid tazopsine (1) together with Sinococuline (2) and two other new related morphinan analogs, 10-epi-tazopsine (3) and 10-epi-tazoside (4). Structures were characterized by 2D NMR, MS, and CD spectral analysis. Compounds 1-3 were found to fully inhibit the rodent P. yoelii liver stage parasites in vitro.
New bisbenzylisoquinolines, fatty acid amidic aporphines, and a protoberberine from Formosan Cocculus orbiculatus.[Pubmed:16038548]
J Nat Prod. 2005 Jul;68(7):1056-60.
Two new bisbenzylisoquinoline alkaloids, (+)-coccuorbiculatine A (2) and (+)-10-hydroxyisotrilobine (3), two new amidic aporphines, a mixture of (+)-laurelliptinhexadecan-1-one (6) and (+)-laurelliptinoctadecan-1-one (7), and one new protoberberine (-)-4-methoxy-13,14-dihydrooxypalmatine (8) have been isolated from the stems of Taiwanese Cocculus orbiculatus. The structures were established on the basis of extensive analysis of spectroscopic data and by comparison with known related metabolites. Cytotoxicity of the isolated compounds was examined toward HepG2, Hep3B, MCF-7, and MDA-MB-231 cancer cell lines. Alkaloids 1 and (-)-Sinococuline (9) showed significant inhibitory activity against the target cell lines.
Cytotoxic effects of sinococuline.[Pubmed:8616827]
Cancer Lett. 1996 Feb 6;99(2):217-24.
Three alkaloids, aknadinine, 1-nitroaknadinine and Sinococuline, isolated from Stephania sutchuenensis were studied for their effects on a fibroblast cell line, eight tumor cell lines and a rat alveolar macrophage culture. Sinococuline is an effective tumor cell growth inhibitor whereas the toxicity of aknadinine and 1-nitroaknadinine towards all tested cells is low. A dose-dependent decrease in cell viability and in the uptake of tritiated-thymidine, -leucine and -uridine by these cells was observed when they were grown in the presence of Sinococuline for 24 h. Exposure to Sinococuline in vitro also altered the macrophage function by reducing the production of tumor necrosis factor and reactive nitrogen intermediates. Human leukaemic HL60 cells and mouse fibroblast L929 cells were used to study the underlying mechanism of cytotoxicity and apoptosis seem to be the mode of death induced by Sinococuline


